During the first half of the 20th century, the crippling toll of poliomyelitis grew alarmingly in Canada while other major childhood disease threats, particularly diphtheria, as discussed in Article #4 of this series, had come under control by the 1930s thanks to Connaught Laboratories’ pioneering work expediting the development, testing and free availability of diphtheria toxoid. Unlike diphtheria, which is caused by a bacterium, poliomyelitis is caused by a virus that only infects humans, and understanding how it infected, how it spread, and how to prevent it, remained major scientific and public health challenges as polio epidemics escalated, especially in North America.

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Soon after the end of World War II, solving the scientific enigma of polio became a primary focus of research and innovation at Connaught Laboratories. This work was supported by several new funding sources, including the National Foundation for Infantile Paralysis (NFIP) in the U.S., which raised funds during its annual “March of Dimes” campaigns. Although polio had also been known as “infantile paralysis,” its threat would expand into older age groups, including adults. The particular challenges of poliovirus research required new virology expertise and virus research facilities that were part of a significant post-war expansion of the Labs that was reflected in a new name, “Connaught Medical Research Laboratories.” Poliomyelitis, in the absence of two main types of vaccines - inactivated poliovirus vaccine (IPV), and attenuated live oral poliovirus vaccine (OPV) - initially infects the gastrointestinal tract, usually harmlessly, but can become more serious if it escapes the gut and, via the bloodstream, enters the nervous system. There it can attack motor neurons in the spinal cord, damaging the connections between the brain and muscles and causing varying degrees of weakness or paralysis. Maternal antibodies provide some natural protection for infants, but the poliovirus can easily spread between people, especially young children, most often via oral-fecal contact. Polio’s most severe effects include paralysis of the muscles that control breathing, leading to the use of the “iron lung.” First developed in the late 1920s, the iron lung was a large tube-shaped negative pressure ventilator which contained the whole body, except the head, and helped a patient breathe. By the early 20th century, polio had become a growing epidemic threat, especially in Europe and North America. As public health and sanitary standards improved, what had been almost universal and naturally immunizing circulation of the poliovirus among infants was steadily reduced, a process most apparent in smaller communities with less population density. Thus, over time, a growing percentage of older children, as well as young adults, particularly among the middle class during the postwar “baby boom,” when they were inevitably exposed to the virus were more vulnerable to it progressing to the nervous system and causing paralytic disease.

[Sanofi Pasteur Canada Archives]

[Library & Archives Canada]
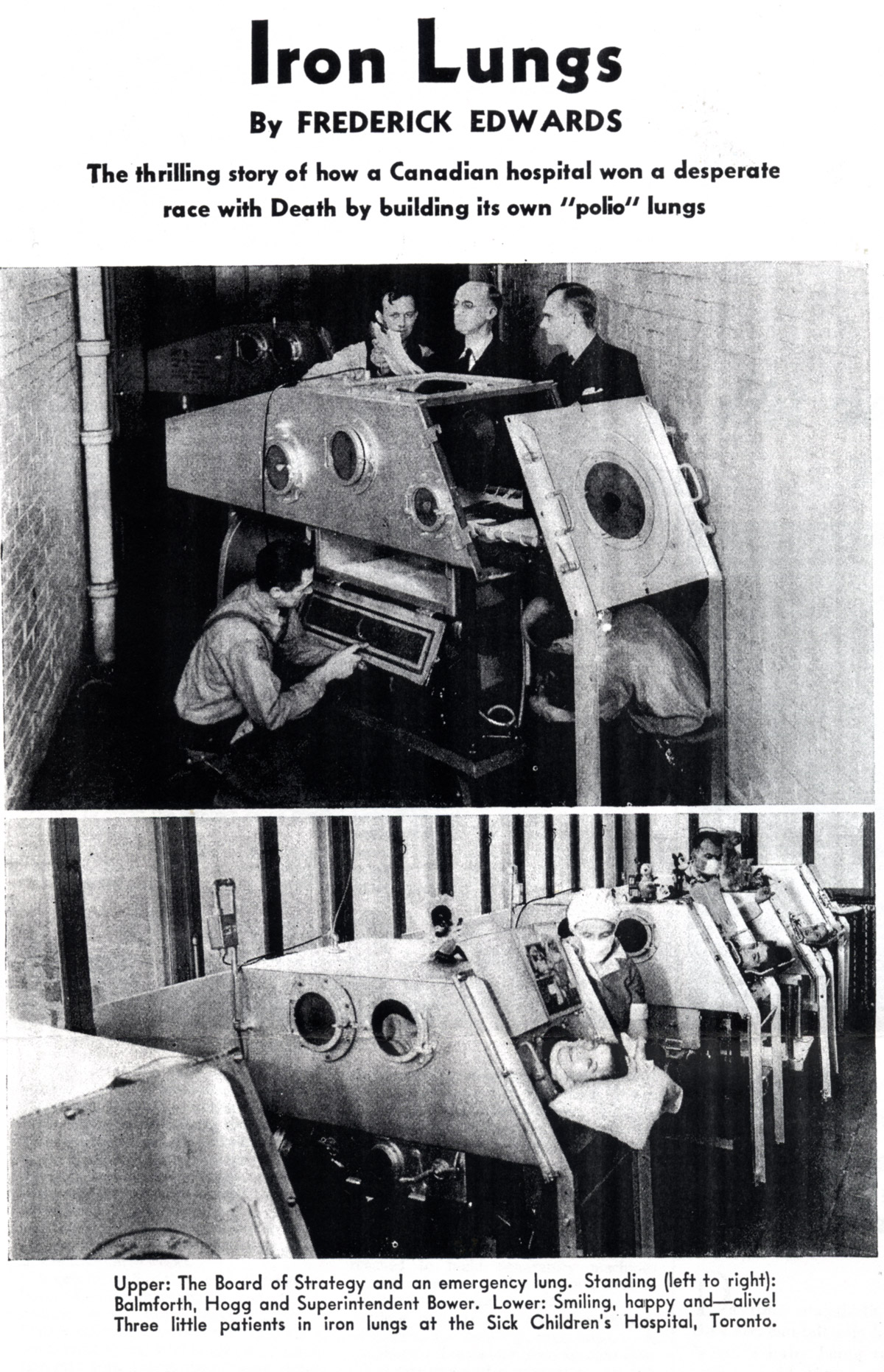
[Maclean’s, Jan. 15, 1938, page 12]
Beginning sporadically in the 1910s, Canada experienced numerous polio outbreaks, with a first significant wave of outbreaks growing larger as the disease struck most provinces in turn each year from west to east, from British Columbia in 1927 through to Quebec in 1932. A second wave of larger polio epidemics hit Manitoba in 1936 and then Saskatchewan and especially Ontario in 1937, reaching new heights of severity with 2,546 cases and 119 deaths in the province. Several provinces were affected during a third wave, particularly Manitoba and New Brunswick in 1941. A fourth polio epidemic wave, however, from 1946 through 1953, became the most serious, ultimately involving all provinces, and even reaching into parts of the Arctic. In 1953, Canada’s worst polio epidemic year, there were a total of almost 9,000 cases and 494 deaths across the country.

[Toronto Star, August 17, 1910, page 1]
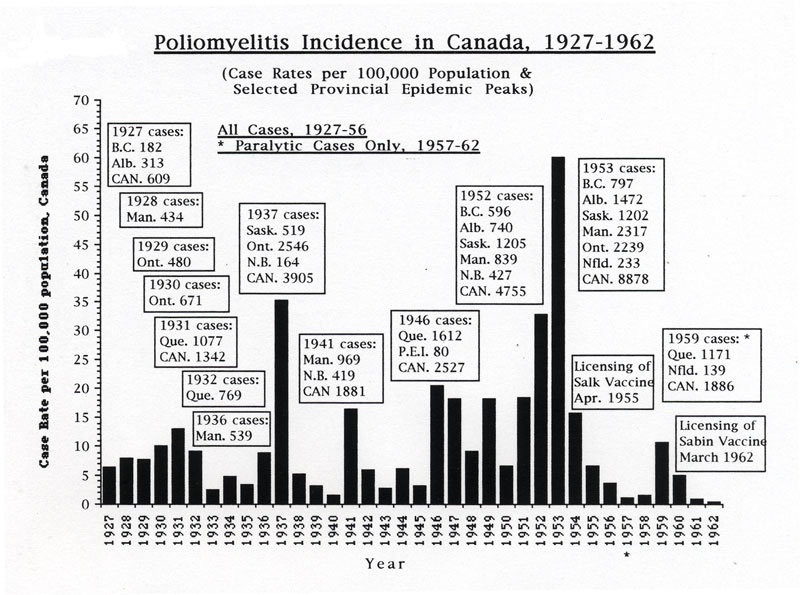
[Rutty, C.J., "'Do Something! Do Anything!' Poliomyelitis in Canada, 1927-1962", Ph.D. Thesis, University of Toronto, 1995, p. 395]
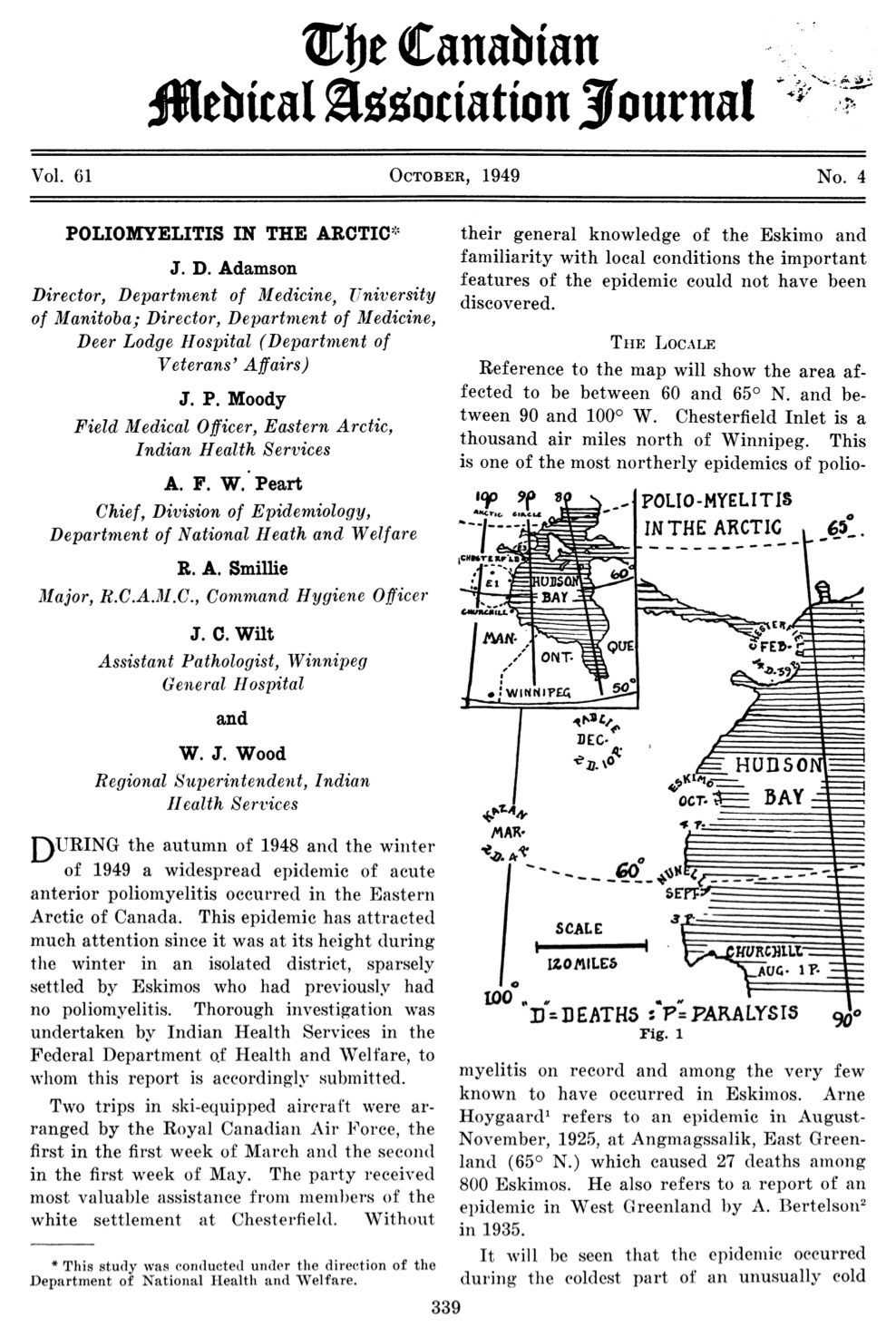
[Canadian Medical Association Journal 61 (Oct. 1949), page 339]

[Sanofi Pasteur Canada Archives]

[Public Domain Image]
In 1946, Dr. Robert D. Defries, Director of Connaught Medical Research Laboratories, began an initiative to recruit productive virus researchers to tackle the growing polio problem. His task was aided by a surge in available polio research funding, particularly from the United States. Directly inspired by U.S. President, Franklin D. Roosevelt’s personal polio experience in 1921 when he was a Senator, the National Foundation for Infantile Paralysis was established in 1938 by FDR and his lawyer, Basil O’Connor, to provide financial support to polio victims and to sponsor well-coordinated research into all aspects of the disease. Connaught had been an early grantee of NFIP funds, but the Labs’ initial polio research work was displaced by its many war-related projects, which were discussed in Article #6 of this series. Defries set his sights on the authors of Virus Diseases of Man, a text book first published in 1940, that quickly became the go-to reference work in the field of virology. First recruited was Dr. Clennel van Rooyen, born in Ceylon and educated in England, who became an authority on virus diseases, especially through wartime experience that brought him face-to-face with outbreaks of typhus, smallpox, hepatitis and polio. Then by June 1947, Defries secured the appointment of Dr. Andrew J. Rhodes, who had become a leading expert on viruses, especially the poliovirus. While working towards his M.D. at the University of Edinburgh, Rhodes met Dr. van Rooyen and they collaborated on Virus Diseases of Man. In 1945, Rhodes had joined the London School of Hygiene and Tropical Medicine, his focus on the poliovirus sparked by the unexpected recognition of polio as an international problem during the war, brought to light by the military’s direct experience with the disease. Incidence of paralytic polio among soldiers serving, in particular, in the Middle East, India, the Philippines, China and Japan, was about 10 times higher than in their home commands. As Rhodes recognized, these troops effectively served as human guinea pigs and drew attention to the presence of poliovirus in communities where paralytic disease did not appear prevalent in the native population; they were immune while many young soldiers had not yet been exposed to the virus were vulnerable paralytic polio. Rhodes’ broadened awareness of polio during the war demonstrated the need for greater systematic poliovirus research and Defries’ offer to join Connaught provided this opportunity.

[Globe & Mail, Sept. 11, 1948, page 3]

[Globe & Mail, Jan. 2, 1948, page 15]

[New York Times, March 9, 1949, page 11]

[Canadian Journal of Public Health 40 (Oct. 1949), p. 418]

[Globe & Mail, Sept. 25, 1951, p. 17]
[Health, April-May 1955, p. 10]
Over the next six years, drawing on new NFIP funds, along with support from the Canadian Life Officers Association and newly established Federal Public Health Research Grants, Rhodes led a productive team of researchers through a series of poliovirus studies. They focused on developing better laboratory methods for the diagnosis of polio, and conducted epidemiological investigations of the poliovirus in rivers and sewage between outbreaks. Perhaps the most dramatic challenge for Rhodes’ research team came when an especially severe polio epidemic struck the Inuit community of Chesterfield Inlet (Igluligaarjuk), Northwest Territories, on the west coast of Hudson Bay. Occurring during the winter of 1948-49, and mostly striking adults, this outbreak challenged contemporary understandings of how polio spread, where and when it could strike, and whom it could infect. In total, there were 60 cases reported among a population of about 275, leaving 38 with paralysis of varying degrees and 13 dead. There were also 25 non-Inuit living in the community, but none appeared affected. The challenge for Rhodes and his team in Toronto was securing from Chesterfield Inlet tissue samples from surviving and fatal cases and confirming in his lab the diagnosis of what appeared to be polio, despite the highly unusual circumstances. Rhodes’ Arctic polio investigations would contribute to a more precise understanding of polio’s epidemiology and its human immunology which echoed in the Inuit stricken by polio in the Arctic what had been revealed among soldiers stricken by the disease in the tropical environments they served in during the war. It was thus clear that polio epidemics had very little to do with geography or a summer “polio season.”

[Sanofi Pasteur Canada Archives]
The insights Rhodes’ team gained from their poliovirus investigations coincided with key advances from researchers in other labs. Of most significance was the discovery in 1949 of a method to cultivate the poliovirus in test tubes using non-nervous cell tissue cultures. This work was conducted by a research team led by Dr. John Enders at Boston Children’s Hospital and would free researchers from a dependence on lab monkeys to grow and test the poliovirus and opened the door to a possible polio vaccine. For this discovery, Enders’ research team was awarded the Nobel Prize in Physiology or Medicine in 1954. Another key advance came in 1951, which confirmed that there were three immunologically distinct types of poliovirus (types I, II and III), this work conducted by Drs. David Bodian and Isabel Morgan at Johns Hopkins University. Building on these breakthroughs, and his own work preparing an inactivated influenza vaccine, Dr. Jonas Salk, of the University of Pittsburgh, developed a precise method for reliably inactivating or killing the poliovirus with formaldehyde while retaining the virus’ ability to stimulate an immune response. The result was an inactivated polio vaccine that proved encouraging in laboratory monkeys. However, a key new element was needed that would make it safe for Salk to proceed with human tests of the vaccine. While the pace of poliovirus research accelerated at Connaught and elsewhere during the late 1940s, it was increasingly clear that better defined nutrient media were needed to feed the cells required for the cultivation of viruses. Existing nutrient media included animal-based ingredients that could not be precisely quantified. Dr. Raymond C. Parker, a cell researcher who joined Connaught in 1941, partnered with biochemist Dr. Joseph F. Morgan and technician Helen Morton, to develop a complex mixture of amino acids, vitamins, iron and various growth factors that would promote cell growth. In 1949, after more than two years and 198 attempts, they finally hit upon a precise blend of 60 ingredients that efficiently promoted continuous cell multiplication in the absence of any blood serum or embryonic extracts. This purely synthetic cell nutrient medium was initially utilized to enable a much clearer understanding of what elements promoted or slowed the growth of cancer cells, and indeed all types of cells; thus was born “Medium 199.” In November 1951, at one of Connaught’s regular staff seminars, Dr. Arthur E. Franklin, a new member of Rhodes’ team, met Dr. Joseph Morgan of the Medium 199 team. Both had recently received a Ph.D. in biochemistry and seemed to have much in common. Franklin told Morgan of the obstacles he was encountering in cultivating the poliovirus in various animal tissues, especially with traditional nutrient media. Morgan suggested Franklin try using “Medium 199” and supplied him with some. It was quickly apparent that this purely synthetic mixture solved, quite spectacularly, most of the problems Franklin was having with cultivating the poliovirus; the yields and purity of poliovirus cultures improved drastically. When Rhodes’ found out about the results, the otherwise unflappable scientist, jumped up on a chair and cheered.

[Sanofi Pasteur Canada Archives]

[Globe & Mail, April 22, 1952, page 13]
News of the success with Medium 199 quickly reached Salk in Pittsburg, who requested a supply from Connaught. This enabled him to prepare the vaccine for its first human use. The first trial took place at a residence for disabled children, most having already contracted polio, to test for antibody response and potential side effects. While Salk completed his initial vaccine trials and the results seemed positive, a problem emerged: how to make the vaccine on a large enough scale for a definitive field trial, and then hopefully for general use? The answer was found by Dr. Leone Norwood Farrell, a researcher at Connaught with experience in the large-scale production of biological products. Farrell, an innovative and pioneering scientist, was one of the few women to earn a Ph.D. in the sciences in the first half of the twentieth century. After a B.A. in Chemistry and a Masters in Zymology in 1929, both at the University of Toronto, Farrell had worked at the National Research Council and then studied at the London School of Hygiene & Tropical Medicine, before completing her Ph.D. in Biochemistry at U of T in 1933. Farrell joined Connaught in 1934 and after work on preparing vaccines to prevent staphylococcus and dysentery, she developed the “Toronto Method” for the deep culture production of specific types of bacteria in a semi-synthetic liquid cell nutrient based on culture bottles that were gently “rocked” on custom designed racks in an incubator room. The traditional method had been to cultivate bacteria on culture plates using solid media, or in still bottles using liquid media. By the early 1940s, Farrell had adapted the “Toronto Method” to the production of pertussis vaccine, and a decade later was asked to do the same for cultivating the poliovirus. Her work was based at Connaught’s Spadina Division (today known as the Daniels Faculty of Architecture at 1 Spadina Crescent), which also accommodated the Labs’ penicillin production facility that was established during World War II, as was described in Article #6 of this series. The “Toronto Method” for poliovirus cultivation used larger bottles than was the case for bacterial cultivation, with 5-litre “Povitsky” culture bottles made of highly durable Pyrex glass proving ideal. The other key difference in the poliovirus cultivation process was the initial culturing in the rocking bottles of a specific type of animal cell tissue (monkey kidney tissue worked best), rather than a specific type of bacteria. The first step in the “Toronto Method” for poliovirus production process was thus to use Medium 199 to maximize the cultivation of monkey kidney cells in the rocked bottles. The poliovirus was then carefully added to each bottle; it would infect and replicate in the kidney cells, eventually killing them, leaving only the live virus in the Medium 199. A precise amount of formaldehyde would then be added to the virus fluids to kill the virus while leaving its distinctive antigenic surface intact. After careful filtration and many tests, the final vaccine was complete.

[Image courtesy of Judy Anderson, grand-neice of Dr. Farrell]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
In early July 1953, Salk’s experience with vaccine trials on a small scale, coupled with Connaught’s progress with developing a large-scale poliovirus production method, convinced the NFIP to sponsor an unprecedented plan for a mass field trial. The NFIP’s commitment to a large field trial followed the highest polio incidence levels yet experienced in the U.S., with 57,879 cases reported in 1952 and 3,145 deaths. The situation was clearly urgent in the U.S., as it was in Canada. In early July 1953, it was clear that high polio incidence was likely in many parts of Canada. A total of 8,878 cases were ultimately reported in 1953 at a case rate of 59.9 per 100,000, which exceeded the record U.S. case rate of 36.9 per 100,000 in 1952. To make a mass polio vaccine field trial possible, Connaught was asked to supply all of the bulk poliovirus fluids necessary, some 3,000 litres, which were shipped to two U.S. pharmaceutical firms for inactivation and final processing in time for the vaccinations to start in April 1954. Also in July 1953, just as Connaught’s work for the mass polio vaccine field trial began, Rhodes left the Labs to accept the position of Research Director at Toronto’s Hospital for Sick Children. For such an unprecedented initiative for the Labs, Dr. Robert D. Defries assumed personal leadership of its management on top of his normal duties as Director of the Labs and of the School of Hygiene. Indeed, this would be a “herculean task,” as Salk later characterized Connaught’s daunting poliovirus production project.[1]

[Canadian Journal of Public Health 46 (July 1955), p. 265]

[Sanofi Pasteur Canada Archives]

[Globe & Mail, April 5, 1954, page 21]

[Time (Canadian Edition), March 29, 1954, cover]
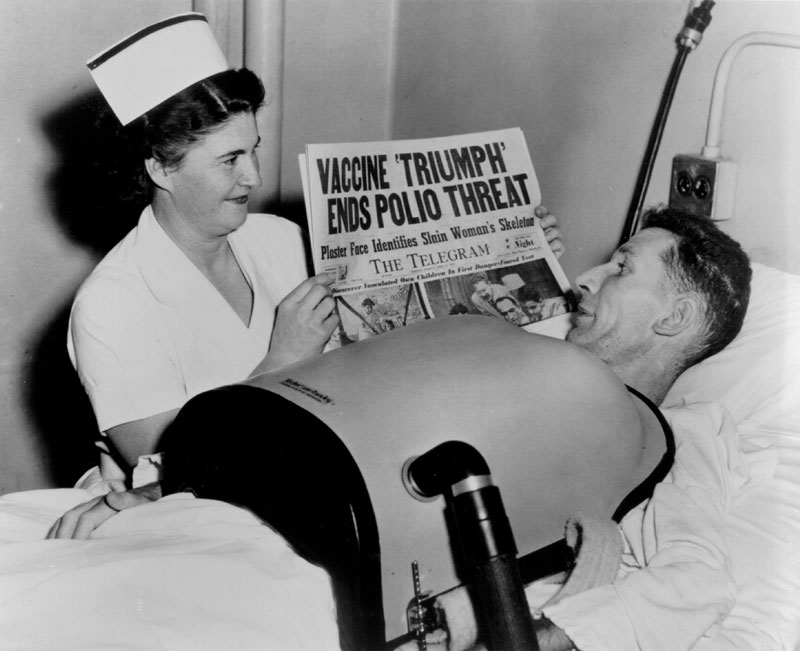
[Photo courtesy of March of Dimes Canada]
The mass polio vaccine field trial was designed to be triple-blind, which meant that neither the subjects, nor the people administering the vaccine, nor the people evaluating the response, knew which subjects were receiving the vaccine or the placebo. The field trial would involve some 1,800,000 “polio pioneer” children in grades one to three in 44 U.S. states. Of this group, some 630,000 were given either the vaccine, or Medium 199 as a placebo, while over 1 million other children were carefully watched to see if they became infected by the poliovirus. Surplus vaccine enabled the extension of the field trial into Alberta, Manitoba and the city of Halifax, as well as the Helsinki area of Finland. In the Canadian trials, 12,456 received three doses of the vaccine and 12,320 were given the placebo, while in Finland, 9,482 children received three doses of the vaccine and 9,309 the placebo. The trial and the complex evaluation of the vaccine’s effectiveness was conducted at the Poliomyelitis Vaccine Evaluation Centre, established at the University of Michigan in Ann Arbor and under the direction of Dr. Thomas Francis. This Centre impartially correlated and analyzed the data collected through the work of many thousands of physicians, health workers and others spread through the 21 participating study areas of the field trial in 44 states. Once the bulk of the virus fluids were produced for the field trial, Connaught focused on preparing the finished vaccine to ensure an anticipated Canadian supply following the announced results of the 1954 field trials. Soon after the trial began, it became clear to Defries that the vaccine was safe (there were no reports of polio cases associated with the vaccine, nor any unusual reactions) and even if the final results showed limited effectiveness, continuing polio epidemics underscored the need for further controlled use and evaluation of the vaccine in Canada to confirm and compare results with the original trial. Thus, Defries worked closely with the federal and provincial governments to plan an all-Canadian observed-controlled trial of the vaccine that would start in April 1955. On April 12, 1955, unprecedented media attention surrounded the long-awaited announcement by Dr. Francis of field trial results. The vaccine proved to be 60-90% effective against the three antigenic types of poliovirus and was immediately licensed for use in U.S. and Canada. In Canada, the vaccine would be distributed through a special federal-provincial program, which provided the vaccine for free to children while its effectiveness was further evaluated.

[Sanofi Pasteur Canada Archives]

[Globe & Mail, Sept. 8, 1955]
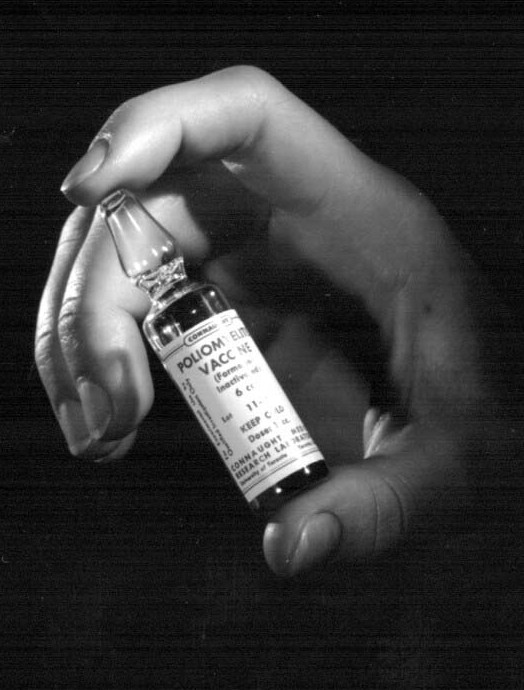
[Sanofi Pasteur Canada Archives]
However, there was a major setback on April 25, when it was discovered that some batches of vaccine from a U.S. producer, Cutter Labs in California, were not fully inactivated, leading to 79 polio cases directly linked to Cutter’s vaccine. On May 7, after first recalling all of Cutter’s vaccine, and then setting up a national polio surveillance system, the U.S. Surgeon General suspended the country’s entire vaccine program.

[Sanofi Pasteur Canada Archives]
North of the border, the burning question was what should Canada do? Despite some resistance from the Prime Minister, Louis St. Laurent, based on the advice of experts and given that there had been no cases of polio linked with Connaught’s vaccine, the only version in use in Canada, the Minister of National Health and Welfare, Paul Martin Sr. (himself a victim of polio, as was his son, future Prime Minster Paul Martin Jr.) decided that Canadian use of the vaccine would continue uninterrupted. With no incidents reported, the Canadian use and evaluation of the vaccine further demonstrated its safety and effectiveness. Canadian political and public health confidence in the vaccine during the “Cutter Crisis” meant a lot to Salk and helped pave the way for the resumption of the U.S. polio immunizations in July 1955. The introduction of the Salk polio vaccine marked the end of an era for Connaught Laboratories. Shortly thereafter, in September 1955, Defries retired from his position as Director of the Labs and of the School of Hygiene. Defries’ remarkable public health legacy was recognized by the American Public Health Association, bestowing on him its highest honour, the Albert Lasker Award, in November 1955. The citation said: “Seldom is there combined in one man great scientific knowledge and judgment, together with the personality and organizing ability to carry through new technical advances to their effective application in the control of disease.”[2] Connaught’s polio vaccine research and innovation work would continue on many fronts under its next Director, Dr. James K.W. Ferguson. During the latter half of the 1950s and into the early 1960s, Connaught scientist provided leadership with improving and expanding polio vaccine production, which brought polio immunization to older age groups. Connaught also played an important role in exporting the vaccine to many countries otherwise without protection against polio. The Labs was also a pioneer in combining polio vaccine with other antigens (diphtheria, tetanus and pertussis vaccines), further broadening polio protection, and also played a key role in the development, evaluation and global distribution of a second type of polio vaccine. Connaught’s polio vaccines story certainly continued after 1955, as will be discussed in the next article in this series.
Useful Resources:
Bator, Paul with Andrew J. Rhodes: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories, Volume 1, 1927-1955 (Ottawa: Canadian Public Health Association, 1990) Defries, Robert D.: The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (University of Toronto Press, 1968) Rutty, Christopher J: “’Do Something! Do Anything!’ Poliomyelitis in Canada, 1927-1962, Ph.D. Thesis, Department of History, University of Toronto, 1995; available here: http://healthheritageresearch.com/clients/docs/Polio-PHD/Rutty-CJ-PolioInCanada-PhDThesis-UT-History-1995-digitalversion.pdf Rutty, Christopher J.: “The Middle Class Plague: Epidemic Polio and the Canadian State, 1937-37,” Canadian Bulletin of Medical History, 13 (1996): 277-314; available here: https://www.utpjournals.press/doi/pdf/10.3138/cbmh.13.2.277 Rutty, Christopher J.: “Dr. Robert D. Defries (1889-1975): Canada’s ‘Mr. Public Health’”, in Lois N. Magner (ed.), Doctors, Nurses and Practitioners (Westport, CT: Greenwood Press, 1997: 62-69; available here: http://healthheritageresearch.com/Defries-biopaper.html Rutty, Christopher J.: “Conquering the Crippler: Canada and the Eradication of Polio,” Canadian Journal of Public Health 96 (March-April 2005): special insert; available here: http://healthheritageresearch.com/ConqueringtheCrippler_e.pdf Rutty, Christopher J. and Sullivan, Susan: This is Public Health: A Canadian History (Canadian Public Health Association, 2010), online eBook: https://www.cpha.ca/history-e-book Rutty, Christopher J.: “Mercy Mission: Fighting Polio in the Arctic,” Canada’s History Magazine, Feb-March 2018, p. 30-37; available here: http://healthheritageresearch.com/clients/docs/Arctic-Polio/RuttyCJ-MercyMissionArcticPolio-CanHistMag-2018-02-03-p30.pdf Sanofi Pasteur Canada, “The Legacy Project”: http://thelegacyproject.ca “Vaccines & Immunization: Epidemics, Prevention & Canadian Innovation: The Online Exhibit, Museum of Health Care, Kingston (2013-14); http://www.museumofhealthcare.ca/explore/exhibits/vaccinations/ “Within Reach of Everyone: The Birth, Maturity & Renewal of Public Health at the University of Toronto,” Dalla Lana School of Public Health website feature: http://www.dlsph.utoronto.ca/history/
Endnotes:
[1] Jonas Salk to Robert D. Defries, January 28, 1954, Sanofi Pasteur Canada Archives, 83-015-05. [2] Paul Bator, with Andrew J. Rhodes: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories, Volume 1, 1927-1955 (Ottawa: Canadian Public Health Association, 1990), p. 178
