On August 17, 1962, smallpox was finally suspected to have infected 14-year-old James “Jimmie” Orr, and was confirmed three days later. He had arrived in Toronto with his parents on August 12, clearly ill, after travelling by air and rail from Brazil via New York City. But despite visible pockmarks on his face, medical checks at U.S. and Canadian customs had cleared Jimmie to continue to Toronto based on a physician’s earlier suspicion of chickenpox before he left Brazil. Orr became the first confirmed case of smallpox in Canada in twenty years.
[Sanofi Pasteur Canada Archives]

[Toronto Star, Aug. 20, 1962]
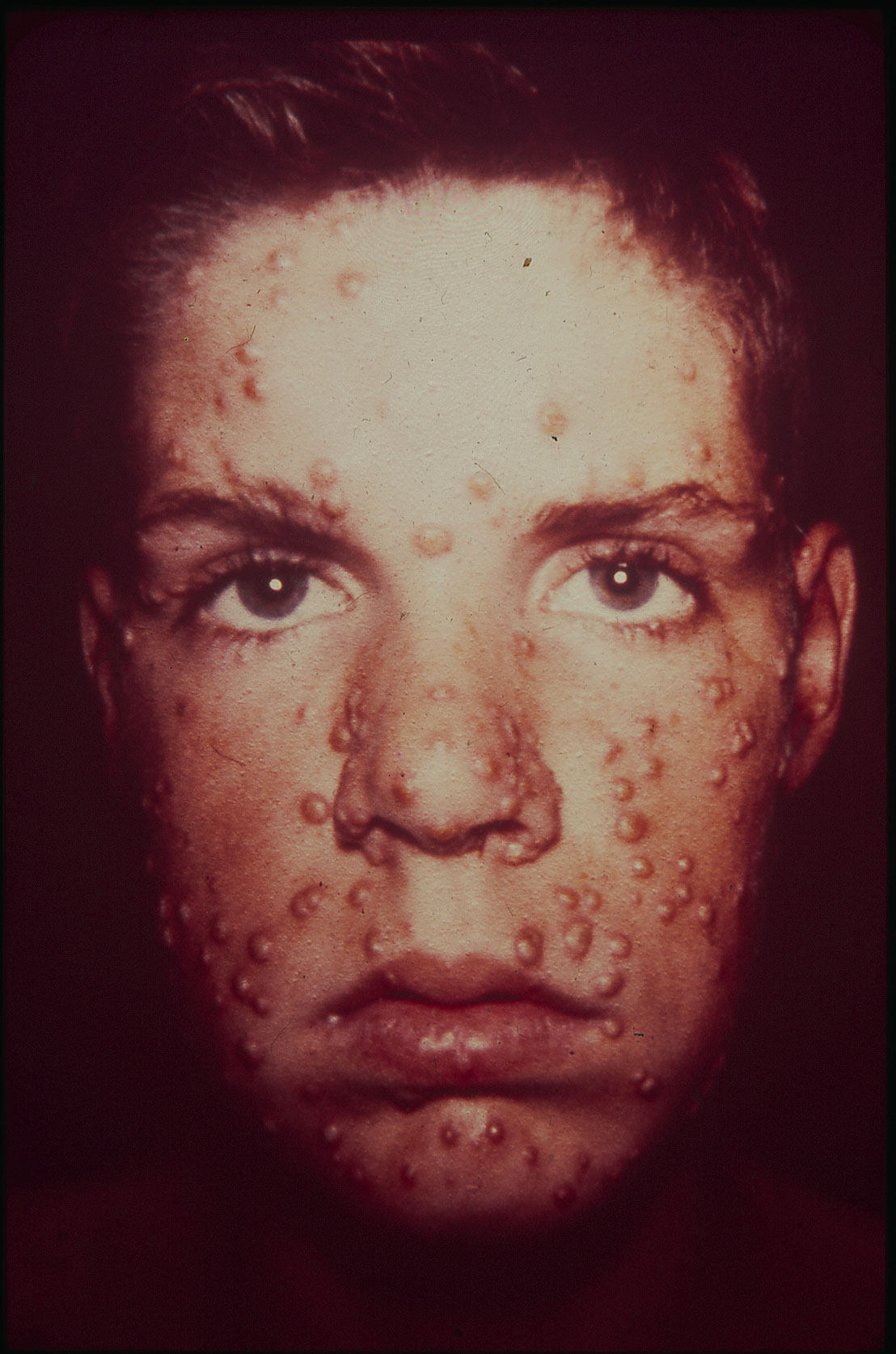
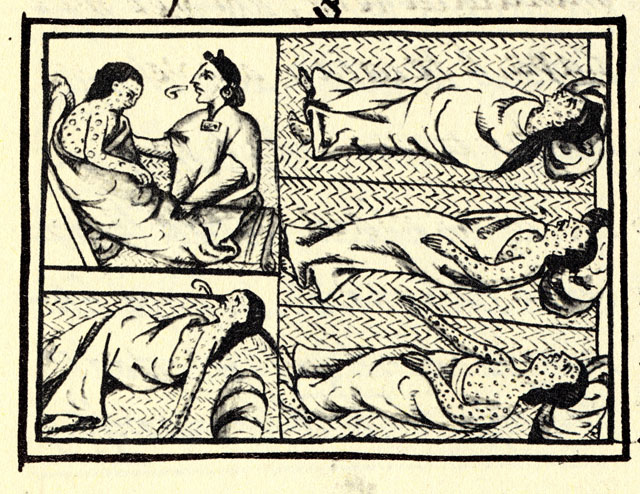
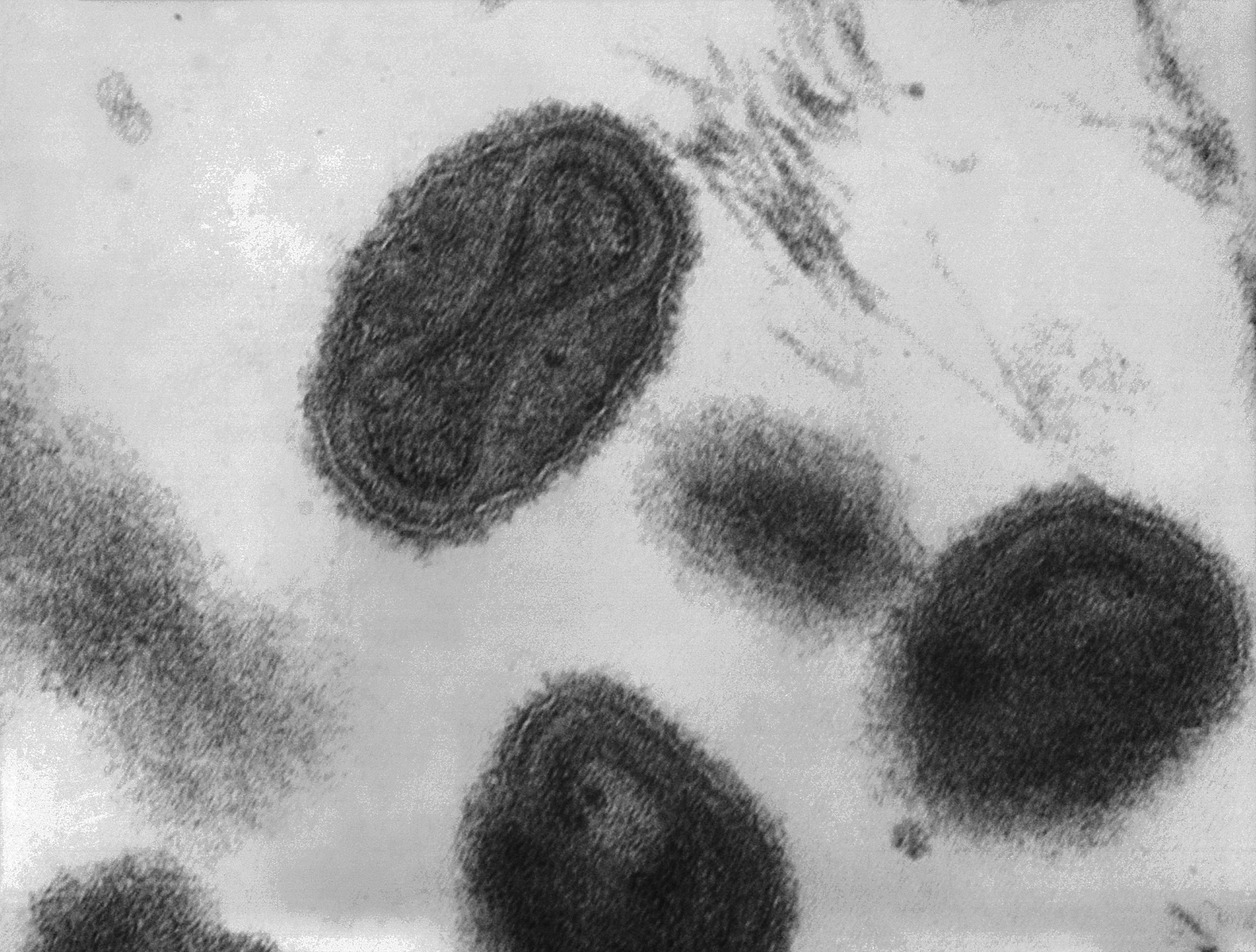
Despite the discovery of smallpox vaccine in 1796, the “Speckled Monster,” as the disease was popularly known, remained a major threat in many parts of the world, including Brazil. Connaught Laboratories had prepared smallpox vaccine since 1916 for Canadian military and domestic use, bringing incidence well under control by the 1940s. Orr’s case, and the potential threat it highlighted in North America, coupled with persistent outbreaks, especially in tropical countries, helped to galvanize renewed smallpox vaccine research and innovation work at Connaught during the 1960s. His case also helped spark renewed international attention and resources, especially from the U.S., to finally eradicate smallpox from the planet. With significant contributions from Connaught, the last smallpox case occurred in 1977, followed by certification in 1979, and formal declaration of smallpox’s death in 1980. Smallpox was a highly contagious infection caused by the Variola virus, with origins going back 10,000 years. Depending on the severity of the strain, death rates among the unvaccinated could range from 30% to 50%. The virus spread from person-to-person through infected droplets from the nose and mouth and direct contact with smallpox rashes and infected blisters. Due to telltale pustules, those who contracted the disease were always left with pockmark scarring on their skin that varied with the severity of infection. The first attempts at smallpox immunization emerged in 11th century China and India through a technique known as variolation. The pus from the pox blister of a mild case was scratched into the skin of a healthy person, which could instill a mild immunizing infection. Variolation became widely used in parts of Europe and North America in the 18th century to control smallpox. The disease had been brought to the Americas by European explorers and settlers starting in the 16th century and spread among indigenous populations with no natural immunity with devastating results; it first appeared in what would become Canada in 1616. However, smallpox variolation was introduced into British North America after the 1763 conquest of New France and was soon offered to indigenous communities.[1]
[Wikimedia Commons]

[UK Science Museum Group]
[Wikimedia Commons]

[University of Toronto Press Journals]
[Photo by Author]
In 1796, English physician Edward Jenner, discovered that exposure to a mild cowpox, or “vaccinia,” infection (vacca is Latin for cow) would effectively immunize people against smallpox. He had noticed that many milkmaids tended to have clear skin, a rarity when smallpox was common. Jenner collected material (“vaccine”) from the skin of cowpox-infected calves, usually on their bellies, and scratched it into the skin of people to “vaccinate” them. The technique was quickly adopted in the British colonies. In 1797, Dr. John Clinch of Newfoundland, a former classmate of Jenner's, asked him to send some vaccine and administered it locally. Smallpox vaccine stations overseen by interested physicians and local health boards and supplied with imported U.S. vaccine, or a local supply, facilitated the distribution of smallpox vaccine in Canada through the mid-1880s. In particular, the Montreal Cowpox Institute was established in 1878. Following one of Canada’s worst smallpox epidemics, which struck the Montreal area in 1885, l’Institut Vaccinogene de Quebec, was established with the support of the Quebec government, while l’Institut Vaccinol de Montreal, a private company, opened in 1899.[2] Modern smallpox vaccine production began in Canada in 1916. That year, the Antitoxin Laboratory (it would become Connaught Antitoxin Laboratories in 1917) purchased the equipment of the Ontario Vaccine Farm. This modest facility had been established in 1885 in Palmerston, Ontario, to produce smallpox vaccine after the Montreal area epidemic encroached into Ontario.[3] Connaught’s decision to start producing the vaccine was prompted by high demand for the vaccine domestically, and especially from the military. During World War I, it was critical that soldiers were vaccinated before being sent to battle zones where the smallpox threat was high. There was a clear need for vaccine in much larger amounts, and of a higher quality, than the Vaccine Farm could meet. The Labs would produce a liquid “glycerinated” vaccine, rather than “vaccine points” harvested directly from calfskin; glycerin was an effective disinfectant, preservative, and a diluent of the vaccinia virus.

[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]
Connaught’s smallpox vaccine production facility occupied a corner section of the Lab’s main building at its new farm site north of Toronto. The purchase and outfitting of the site was sponsored by distiller and philanthropist, Albert E. Gooderham, who, as described in Article #2 of this series, hoped to help the war effort by financing the expansion of the Lab’s tetanus antitoxin production capacity. The smallpox facility had four rooms, one including a special operating table used to facilitate the inoculation of vaccinia virus on the belly of calves. After inoculation, the resultant infective vesicles on the skin were harvested and processed into glycerinated vaccine.
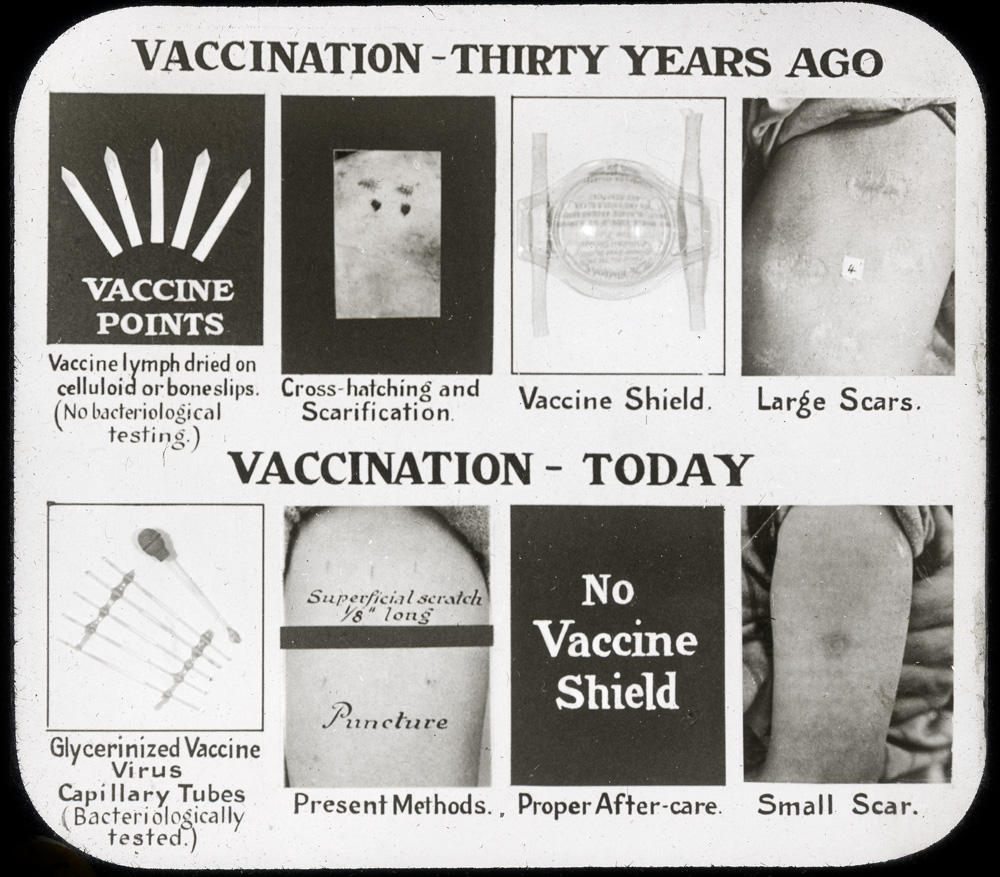
[Sanofi Pasteur Canada Archives]
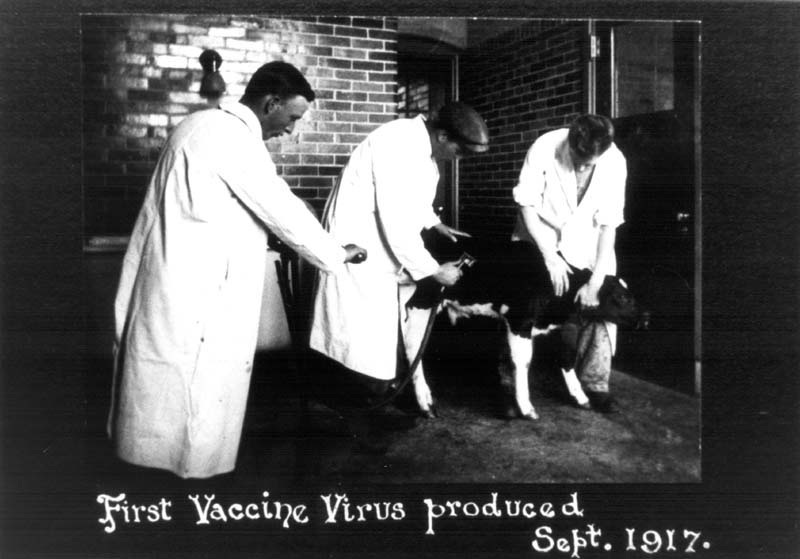
While Connaught’s smallpox vaccine facility was being prepared, and to meet urgent military demand, an arrangement was made with the New York City Department of Health Laboratories to provide a bulk supply of vaccine that was tested and filled at Connaught. The New York City Lab also provided a seed strain of vaccinia virus that had originated in England and which Connaught would use for its vaccine production. With Connaught’s facility finally complete, the Labs’ first smallpox vaccine was harvested in September 1917, and after processing, released for distribution. Production rose sharply thereafter, spurred by high military demand through the end of the war, and also several major smallpox outbreaks in Ontario, especially during 1919-21, when Connaught produced some 1 million doses.[4] This high level of production provided a valuable opportunity for Connaught scientists to not only build up practical experience preparing the vaccine, but also to study its effectiveness, investigate occasional vaccine failures or complications, and to focus on improving its quality.
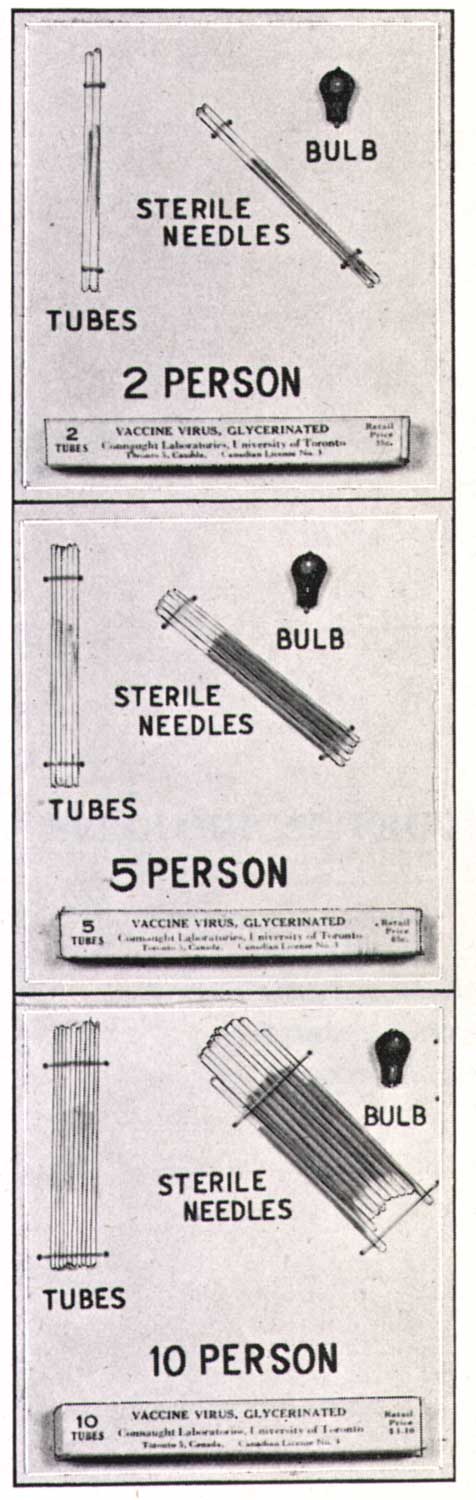
[Sanofi Pasteur Canada Archives]
During the inter-war years, while Connaught met national demand for routine smallpox immunization, several epidemic emergencies in Canada, and also abroad, forced sharp boosts in vaccine production. In particular, especially virulent smallpox epidemics struck in the Windsor area in 1924, and in Vancouver in 1932. During the former crisis the Labs prepared an additional 90,000 doses, and during the latter, enough vaccine was sent to vaccinate 80,000 people.[5] In 1938, China faced a major outbreak of smallpox and made an international appeal for vaccine through the League of Nations. The Canadian government responded and contracted Connaught to supply 1 million doses. In early 1946, a smallpox outbreak in Seattle closed the Canadian-US border to prevent contagion and drove demand for the vaccine in British Columbia. The province’s vaccine supply was quickly exhausted, prompting an urgent vaccine order to Connaught from the B.C. government. The Labs was quickly able to boost production and ship enough vaccine for over 250,000 people.[6] After World War II, a resurgence of smallpox in the developing world refocused international efforts on addressing the disease and new interest in improving vaccine. In 1953, the World Health Organization's first director-general, Dr. Brock Chisholm, previously Canada's Deputy Minister of Pensions and National Health, suggested for the first time that the global eradication of smallpox was possible. After initially rejecting the idea as impractical, the WHO launched a limited smallpox eradication campaign in 1959, which was focused on mass vaccinations in several countries, but dependent upon donated funds and vaccine. By the late 1950s, the Labs’ leadership saw an expanding international market for smallpox vaccine, a market opened up by the growth of its Salk polio vaccine production, as described in Article #8 of this series. There was also a steadily growing domestic market based on an increasing population, a renewed program of re-vaccinations and expanded use among military personnel. Between 1952 and 1958, Connaught's annual smallpox vaccine distribution in Canada grew from about 790,000 to 1,400,000 doses, and by late 1958, the Labs were producing 100,000 doses per month.[7] Connaught had no real competition for its vaccines within Canada, except from the Institut de microbiologie et d'hygiène at the Université de Montréal, which began preparing smallpox vaccine in 1939, but primarily for distribution in Quebec.[8] However, Connaught faced several large commercial competitors in the global smallpox vaccine market, particularly as several companies, led by the Lister Institute in England, had begun developing a freeze-dried type of vaccine, building on technology used to prepare dried blood serum during World War II. There were also research efforts focused on cultivating the vaccinia virus using tissue culture methods along similar lines as the poliovirus.
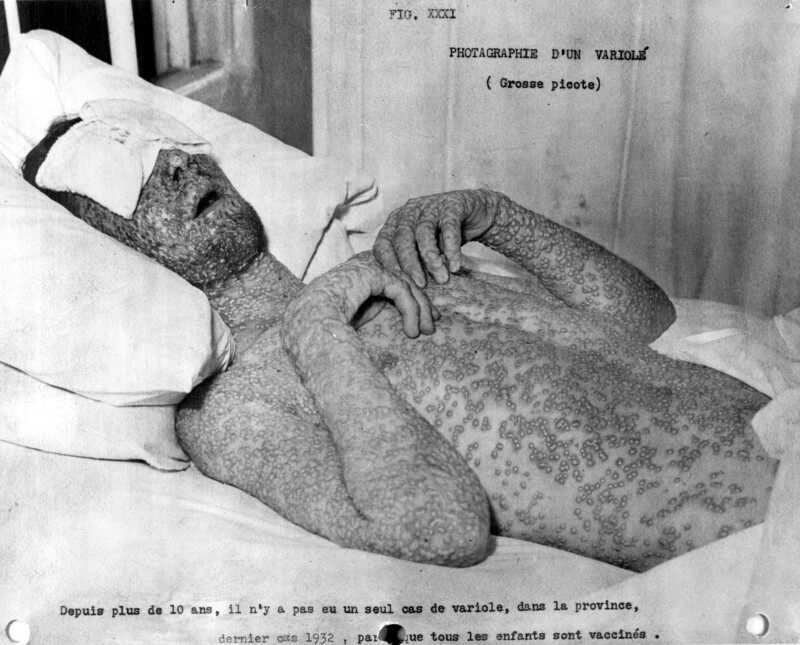
[Sanofi Pasteur Canada Archives]
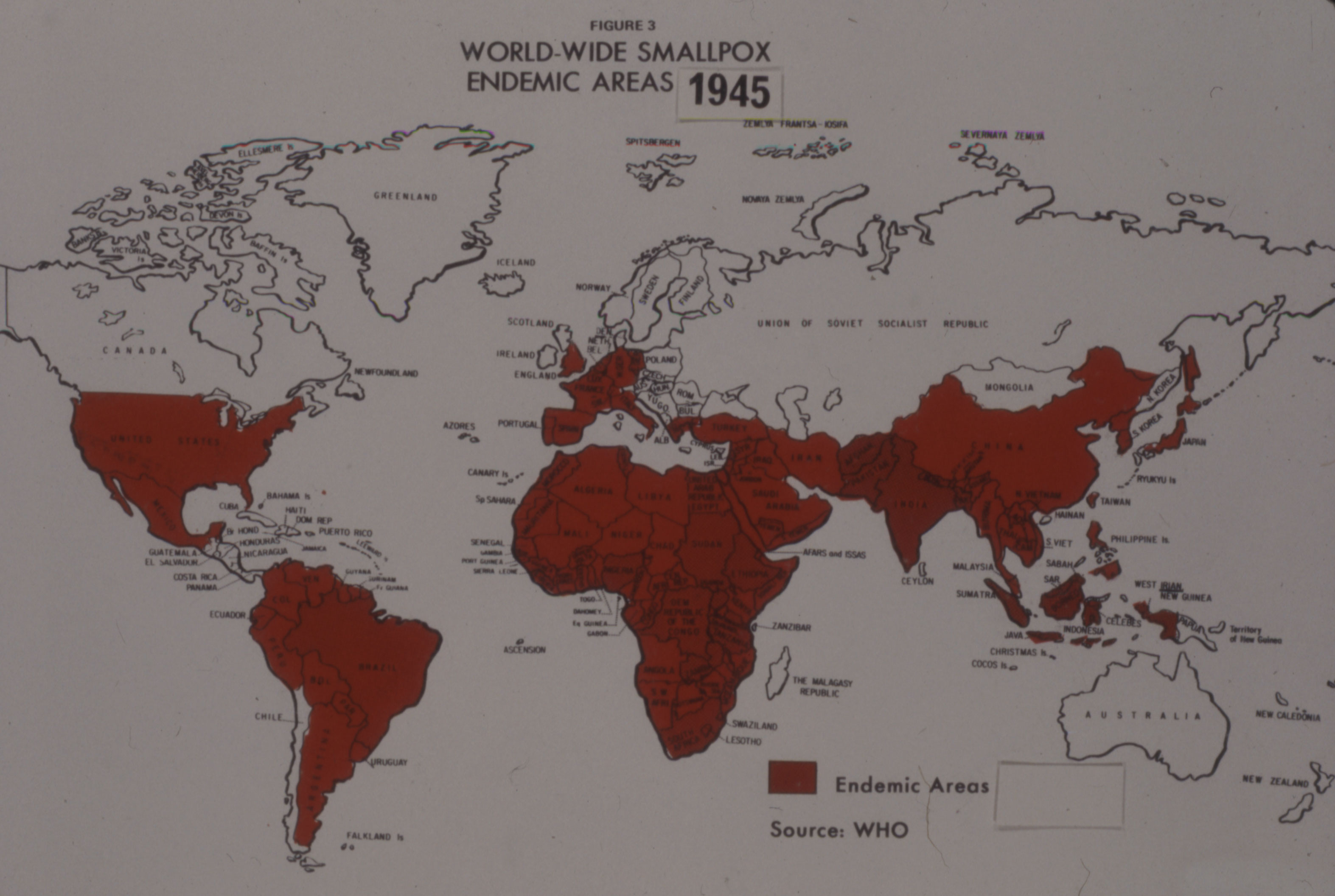
[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]

[Globe & Mail, April 22, 1958, p. 30]
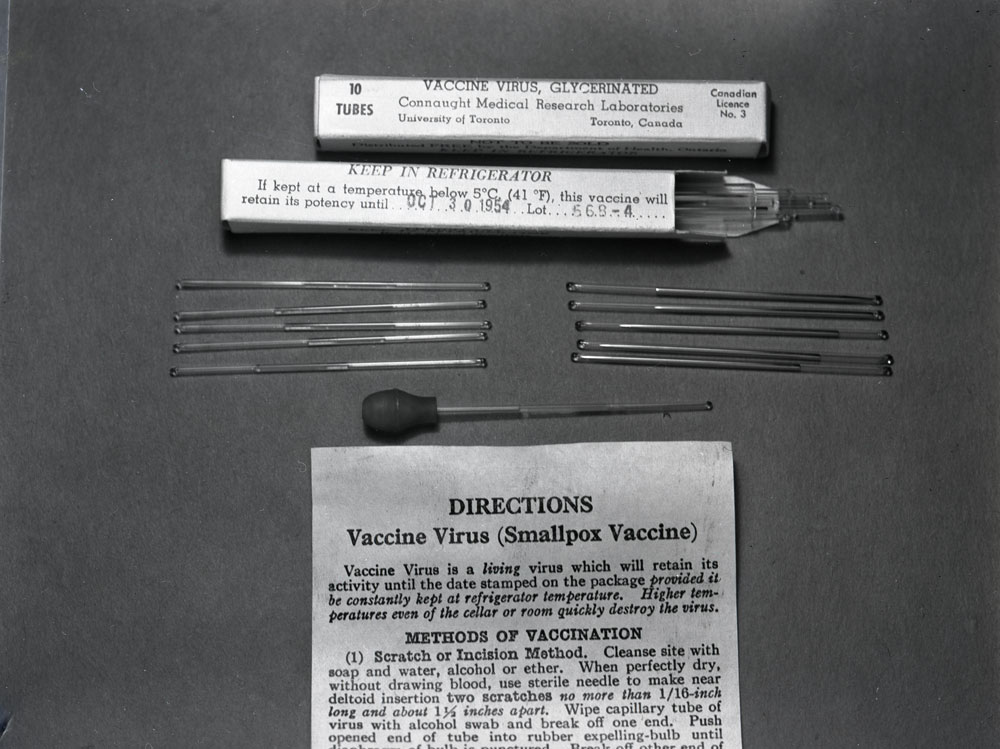
In 1958, a major smallpox epidemic struck in what is today Bangladesh (but then known as eastern Pakistan) resulting in more than 100,000 cases and an urgent call for vaccine from the International Red Cross. Connaught was quickly able to deliver enough vaccine for 1.5 million people. Though effective in helping control this epidemic, Connaught’s standard glycerinated liquid vaccine had a limited shelf life in tropical conditions. A freeze-dried vaccine would retain its potency much longer and thus would ultimately prove the better option to control and prevent similar epidemics. In the fall of 1958, Connaught thus began work on developing a dried vaccine after hiring Dr. Cleeve R. Amies, who had worked at the Lister Institute. Before he arrived, a research effort had been initiated at Connaught to investigate cultivating vaccinia virus in tissue culture in order to prepare an inactivated smallpox vaccine. Although this work was promising, Amies arrival led to a research program focused on the more immediate promise of preparing a dried vaccine. By 1962, Amies had made considerable progress, leading to several dried vaccine clinical trials, particularly in collaboration with the Canadian Armed Forces, which took place in the West Indies and Africa. The dried vaccine was primarily designed for use by the Canadian military, but would also be of value for general export, emergency stockpiles, and for the WHO’s fledgling smallpox eradication efforts. However, Amies left Connaught in early 1962, a move that shifted the Labs’ further development of smallpox vaccines into the capable hands of Dr. Paul Fenje, who had also joined Connaught in 1958.[9] Born in 1915 in Novi Sad, Serbia, Fenje studied medicine, public health and virology before being appointed Head of the Department of Medical Virology at the Pasteur Institute in Novi Sad in 1955. Fenje had endured considerable hardship during World War II, including conscription by the Yugoslavian army as a medical officer, capture by the Hungarian army to work as a prisoner-physician, then capture by the German SS to work as a slave laborer, followed by time in a concentration camp. After the war, Novi Sad became part of Yugoslavia and came under Communist rule, with Fenje forced to work as the only rural doctor for 100,000 people. By 1958, although settled into mostly desk-based work the Pasteur Institute, Fenje hoped to escape the country. Through some “conspiratorial work,” as he recalled, an escape plan developed. He met a Scottish physician who arranged for Fenje to attend a conference in Edinburgh. Meanwhile, Fenje arranged for his wife and two sons to visit her cousin in Vienna, but instead go to London. Fenje’s brother-in-law, who had earlier left Europe for Vancouver, then funded the Fenje family’s steamship ticket to Montreal.[10]

[Toronto Star, Aug. 20, 1962, p. 2]

[Sanofi Pasteur Canada Archives]
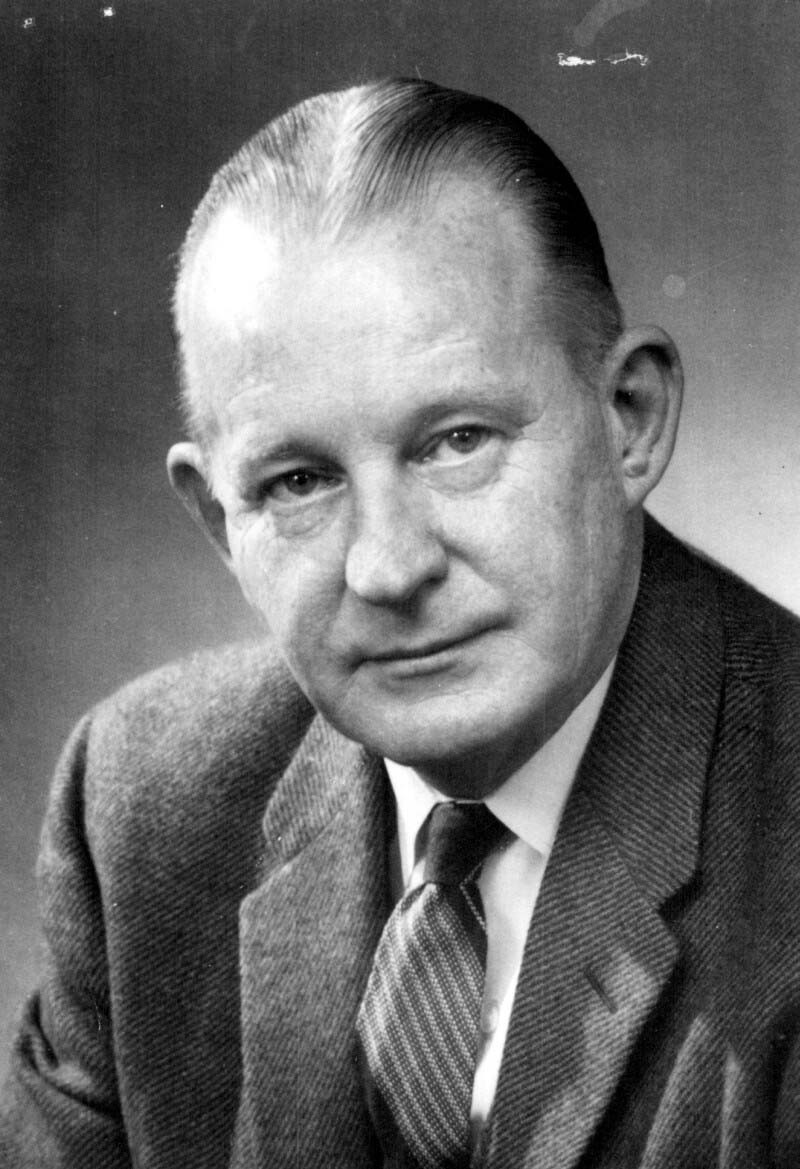
In need of employment, Fenje first visited the Institut de microbiologie et d'hygiène at the Université de Montréal, but they could not use him. He then went to Toronto and met Dr. J.K.W. Ferguson, Connaught’s Director, who was immediately impressed with Fenje’s resume and his language dexterity (he spoke 9 languages). The Labs had an immediate need for a virologist experienced with rabies vaccines to help stem a major rabies outbreak in Ontario and Ferguson hired Fenje on the spot. Fenje would head a pioneering rabies vaccine research effort that would lead to the development of a new generation of tissue culture based rabies vaccines for human and veterinary use.[11] However, in January 1962, after Amies’ departure, Fenje’s work would also include leading the Labs’ smallpox research and production program. While he focused on improving the liquid smallpox vaccine, Fenje spent most of his energy on the further development of a freeze-dried vaccine. Just as Fenje was intensifying his smallpox vaccine work, Jimmie Orr returned to Toronto with smallpox. Fortunately for Orr, it proved to be a mild case and he recovered. But public health authorities in Canada and United States were terrified about the potential of an outbreak. There had been significant outbreaks in Great Britain and in Germany the previous year. As with Orr, the disease had been spread by travelers from places with a higher incidence of smallpox, such as India, Pakistan, and Liberia. The increasing use of international air travel, in particular, raised the risks of importing the virus into regions, such as Europe and North America, where long periods of lack of exposure meant there were low levels of smallpox immunity. After Orr’s smallpox diagnosis, extensive efforts were made to trace any possible person who might have had contact with him from when he arrived in New York City; there were potentially thousands, and as a precaution, vaccination stations were set up in Ontario and New York. According to Dr. Donald Henderson, then of the U.S. Centers for Disease Control, and later director of the WHO’s smallpox eradication program, it had been “all but decided” to vaccinate everyone from New York City to Buffalo, and to close the Canadian border. “Fortunately,” he recalled, “reason prevailed – although just barely – but these events speak for themselves of the fear which smallpox engendered.”[12] In the end, no smallpox cases were discovered among Orr’s contacts.[13] Fenje’s research work on smallpox vaccines at Connaught was also driven by international vaccine shortages and persistent smallpox outbreaks in the developing world. The WHO also put pressure on government biologics regulators to establish higher smallpox vaccine standards after large amounts of Russian vaccine was found contaminated with extraneous viruses. For Fenje, the wide variation in the quality of dried vaccines was of particular concern, as was the levels of bacterial contamination, and how much, or how little, vaccine producers did about it.[14] For Connaught’s Human Antigens Committee, as stressed at its February 21, 1963 meeting, there was a clear commitment that the Labs “should remain in the forefront of the work leading to the best available smallpox vaccine on a production scale.”[15]

[Globe & Mail, Jan. 15, 1962]

[Canadian Journal of Public Health 55 (Aug. 1964), p. 346]
Connaught’s Assistant Director, Dr. Robert J. Wilson, would play a key leadership role in the development of the Labs’ smallpox vaccine program, especially on the international level, building on connections that had grown through Connaught’s leadership with producing and exporting the Salk and Sabin polio vaccines. Born in in Edmonton in 1915 (the same year as Fenje), Wilson earned an M.D from the University of Toronto and a Diploma in Public Health at the School of Hygiene. He was appointed to Connaught in 1946. Among several research interests, as discussed in Article #8, Wilson focused on the development of combined vaccines, particularly DPT-Polio, which was introduced in 1959. In June 1965, Wilson received a phone call from Dr. Donald Henderson, now at the WHO, that U.S. President Lyndon Johnson had pledged considerable financial support to intensify the WHO’s smallpox eradication program.[16] Henderson, who had only seen 13 cases of smallpox before, including Jimmie Orr, was “abruptly” dispatched to Geneva to assume the position of global director for smallpox eradication. Henderson’ limited direct experience with smallpox reflected very low U.S. incidence since the late 1940s, but there was family experience with the disease. His parents were Canadian and his mother worked as a nurse during the major Windsor smallpox epidemic in 1924. Henderson, through his position at the U.S. Center for Disease Control, had built a close relationship with Connaught, and particularly Wilson, during the introduction of both polio vaccines, and worked closely with Wilson and Fenje to re-energize the eradication initiative. He later recalled, “I appreciate only too well how many of the concepts in the execution of the smallpox program saw the first light of day over a glass of beer with Bob and Paul.” “What I don’t recall,” he continued, “is whether the ideas stemmed from Wilson or Fenje, so perhaps they are better attributed to Wilje (or should it be Fenson?)”[17] Wilson suggested that Connaught assume a regional responsibility for the smallpox eradication effort in Latin America, especially Brazil. Connaught’s formal involvement with the WHO’s smallpox eradication program began in January 1967, with Fenje and Wilson visiting some 15 labs in 12 countries across Latin America during the year. Their primary focus was Brazil, where almost all smallpox cases in the region occurred. The Instituto Oswaldo Cruz was the major vaccine producer in Brazil, but the quality of its vaccine was problematic. Its director visited Connaught to study production and testing methods in order to meet the WHO’s vaccine production standards.[18] Fenje and Wilson conducted follow-up visits to local production labs, providing further consultation services to overcome production problems and test batches. As Henderson noted, Fenje and Wilson thus established “a full service vaccine production back-up for the Americas,”[19] ensuring high-quality local vaccine supplies. By this time, the WHO’s vaccine production standards had been codified in its document, Methodology of Freeze-dried Smallpox Vaccine Production,[20] which was based largely on Connaught’s experience and Wilson and Fenje’s initiative. By the fall of 1968, five of the major vaccine producers in South America were meeting, or almost meeting, the standards of adequate potency, stability and bacteriological sterility, often, as Wilson noted to Henderson, “in spite of economic, political and administrative chaos.”[21]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]
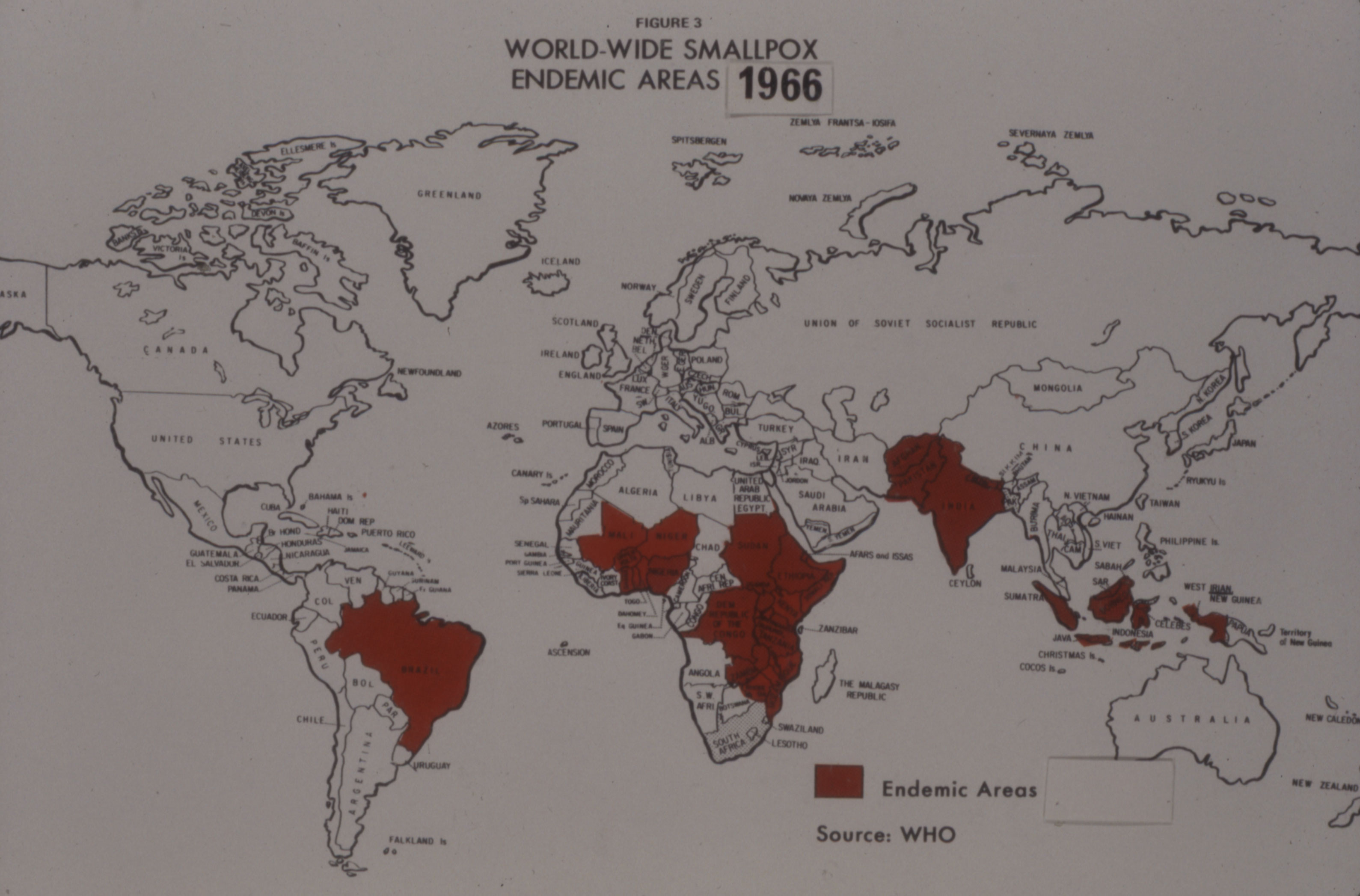
In 1969, based on the success of this initial work, Connaught was formally designated as the WHO Regional Reference Centre for Smallpox Vaccine in the Region of the Americas; the National Institute of Public Health in Bilthoven, Netherlands, would serve as the Reference Centre for the rest of the world. In addition to advising local producers and testing their vaccines, both Reference Centres collected and studied vaccinia virus strains, provided seed virus and reference vaccine when needed, researched vaccine production and testing improvements, and trained virologists in vaccine production and testing.[22]

[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]
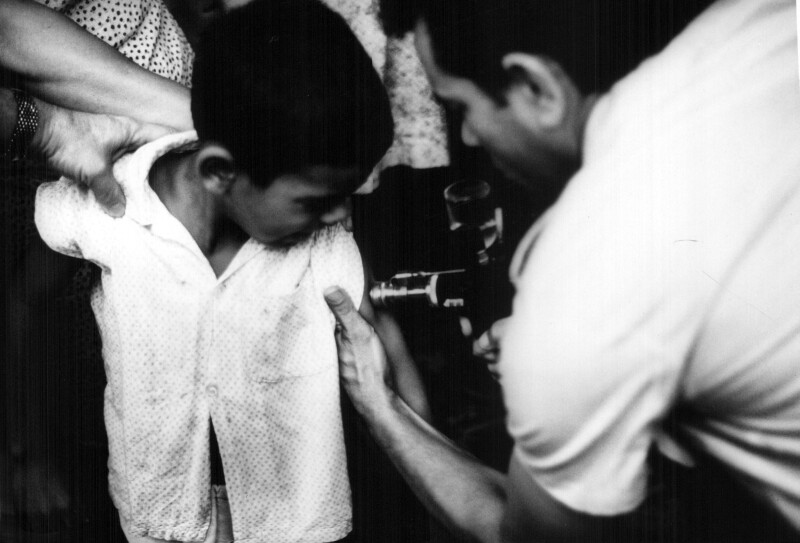
Due to administrative and political complications, the Canadian government was unable to directly donate Connaught’s vaccine to the eradication effort. The main stumbling block was a federal policy in which Canada supported the WHO based on an assessed share of its global budget, which was 2.7% per year, or about $80,000, of the smallpox budget. In January 1968, Connaught’s Director appealed to the Secretary of State for External Affairs, Paul Martin, who had a close relationship with Connaught while Minister of National Health and Welfare (1946-1957), especially during the launch of the Salk polio vaccine. Martin’s hands were tied, particularly as he would run, but loose, as a Liberal leadership candidate. Martin’s subsequent appointment to the Senate and a federal election further complicated the process. In May 1970, however, Connaught got a boost from some unexpected media attention. In particular, Henderson gave “an eloquent presentation”[23] on television about the need for smallpox eradication and for vaccine. The Canadian Mission to the United Nations had approached him to discuss vaccine donations and was troubled about the Canadian government’s restrictions for a direct vaccine donation. Henderson underscored multilateral donations were the most flexible, but the Canadian policy was bilateral, and that the vaccine only cost one cent per dose. At about the same time, Wilson happened to meet a TV producer to whom he pointed out the “stupidity of the Canadian government over a donation of smallpox vaccine.”[24] A TV interview with Henderson was then arranged and shortly after his appearance, the Canadian government committed $140,000 for an initial donation of some 7 million doses of Connaught’s vaccine to the WHO. The total donation would total 35,000,000 doses spread over five years, directed to several African countries, as well as Bangladesh, India, Iran and Pakistan.
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
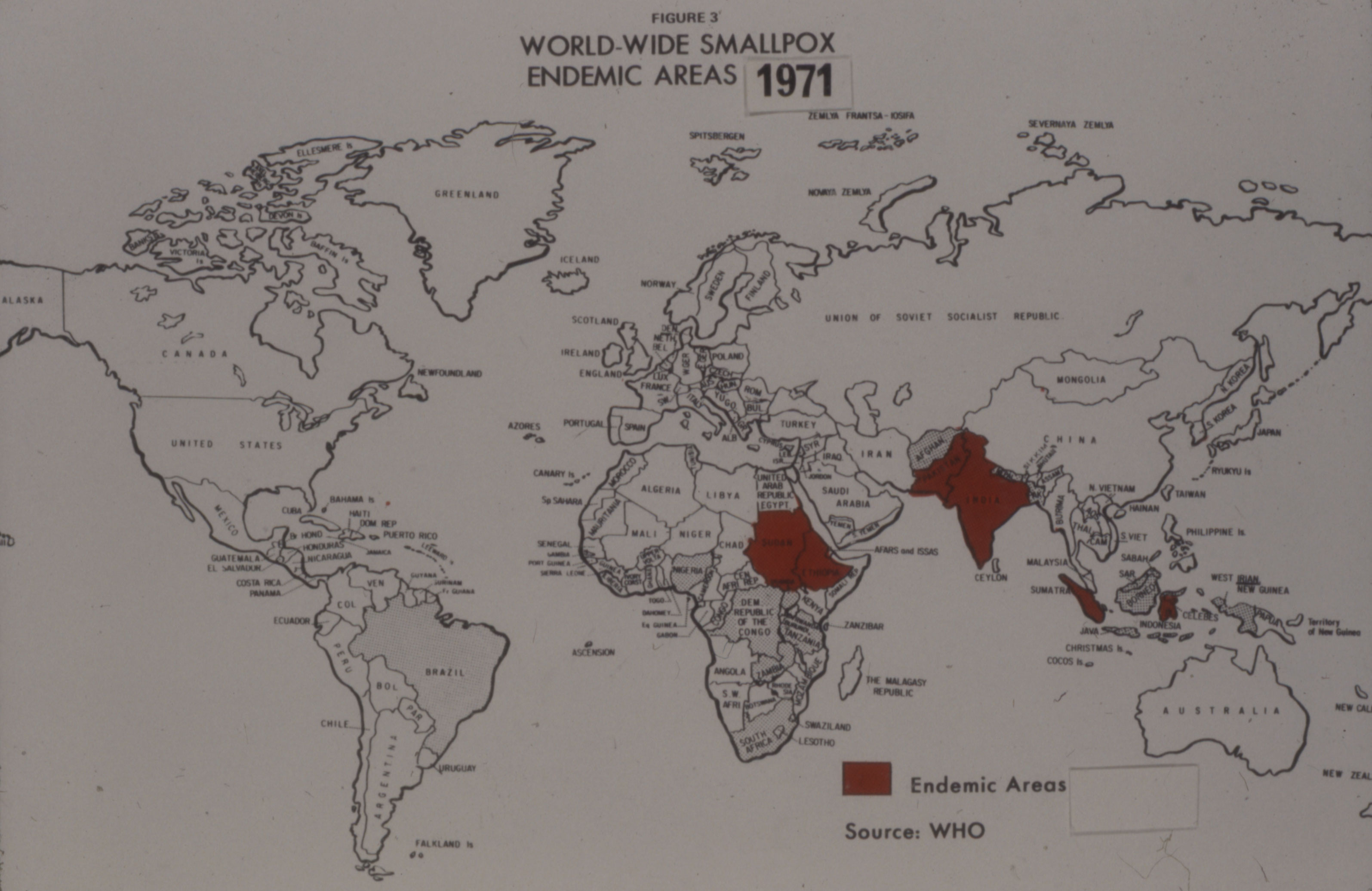
For Fenje, keeping up with the WHO’s demand for vaccine was a major challenge. In February 1971, Henderson was desperate for vaccine, especially in the Congo, and often expressed a preference for Connaught’s vaccine. When Fenje completed the order for 10 million doses, he told Henderson that he would soon have “the best dried smallpox vaccine ever made in our galaxy.”[25] The influx of vaccine meant Henderson could expand his efforts to the Sudan and Ethiopia.[26] Though colourful in his characterization, Fenje was quite serious in his claim of galactic supremacy of his dried smallpox vaccine. Connaught’s ability to consistently prepare a sterile dried vaccine, as well as sterile liquid vaccine, greatly impressed the Canadian and U.S. regulators and the WHO.[27] Despite a major smallpox epidemic in Bangladesh the spring of 1972, and an outbreak in Yugoslavia sparked by a pilgrim returning from Mecca, global smallpox incidence had fallen sharply by the early 1970s. “The Speckled Monster’s” last stand was in Somalia in 1977, the last naturally occurring case confirmed on October 26, 1977. It was a mild case that struck Ali Maow Maalin, a hospital cook, who had volunteered as a vaccinator for the eradication team, but who had ironically never had himself vaccinated. “I was scared of being vaccinated then. It looked like the shot hurt,” he later confessed.[28] Connaught’s freeze-dried smallpox vaccine, and more importantly, the high standard Connaught established for the vaccine, which was adopted by the WHO for all producers, proved critical to reducing global smallpox incidence to its last case in 1977. Indeed, Connaught’s smallpox vaccine story, especially from the late 1950s through the early 1970s, was driven by an intense research and innovation program focused on refining and improving the vaccine. As will be discussed in the next article of this series, a very similar research and innovation program was undertaken during this same period that was focused on the refinement and improvement of bacterial vaccines, especially to prevent diphtheria and pertussis.

[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]

[World Health May 1980]
Useful Resources:
Bator, Paul: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories Limited, Volume 2, 1955-1975; With An Update to the 1990s (Ottawa: Canadian Public Health Association, 1995) Bliss, Michael: Plague: A Story of Smallpox in Montreal (Toronto: Harper Collins, 1991) Defries, Robert D.: The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (Toronto: University of Toronto Press, 1968) Fenje, Paul: “Advances in the Immunoprophylaxis of Smallpox,” Canadian Journal of Public Health 55 (8) (Aug. 1964): 346-52 Fenner, F., Henderson, D.A., Arita, I, Jezek, Z., Ladnyi, I.D., Smallpox and its Eradication (Geneva: World Health Organization, 1988); available here: http://apps.who.int/iris/handle/10665/39485 Jarvis, Eric: “A Contagious Journey Within a Culture of Complacency: The Smallpox Scare of 1962 in New York and Toronto,” Canadian Bulletin of Medical History 24 (Fall 2007): 343-66; available at: https://www.utpjournals.press/doi/abs/10.3138/cbmh.24.2.343 Barreto, Luis and Rutty, Christopher J. : "The Speckled Monster: Canada, Smallpox and its Eradication," Canadian Journal of Public Health 93 (July-Aug 2002): I-1 - I-20 Rutty, Christopher J.: “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” in Kenton Kroker, Jennifer Keelan and Pauline M.H. Mazumdar (eds.) Crafting Immunity: Working Histories of Clinical Immunology (Hampshire, UK: Ashgate, 2008), pp. 273-300; available here: http://healthheritageresearch.com/13-rutty.pdf Rutty, Christopher J. and Sullivan, Susan: This is Public Health: A Canadian History (Canadian Public Health Association, 2010), online eBook: https://www.cpha.ca/history-e-book Rutty, Christopher J.: “A Pox On Our Nation,” Canada’s History (Feb-March 2015): 28-35 Sanofi Pasteur Canada, “The Legacy Project”: http://thelegacyproject.ca Spaulding, William B.: “The Ontario Vaccine Farm,” Canadian Bulletin of Medical History 6 (Spring 1989): 45-56; available at: https://www.utpjournals.press/doi/abs/10.3138/cbmh.6.1.45 “Vaccines & Immunization: Epidemics, Prevention & Canadian Innovation: The Online Exhibit, Museum of Health Care, Kingston (2013-14); http://www.museumofhealthcare.ca/explore/exhibits/vaccinations/ “Within Reach of Everyone: The Birth, Maturity & Renewal of Public Health at the University of Toronto,” Dalla Lana School of Public Health website feature: http://www.dlsph.utoronto.ca/history/
Endnotes:
[1] Christopher J. Rutty, “A Pox On Our Nation,” Canada’s History (Feb-March 2015): 28-35 [2] Luis Barreto and Christopher J. Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," Canadian Journal of Public Health 93 (July-Aug 2002): I-1 - I-20; Pierrick Malissard, “Pharming » a l’ancienne: les fermes vaccinales canadiennes,” Canadian Historical Review 85 (1) (March 2004): 1-16 [3] William B. Spaulding, “The Ontario Vaccine Farm,” Canadian Bulletin of Medical History 6 (Spring 1989): 45-56; available at: https://www.utpjournals.press/doi/abs/10.3138/cbmh.6.1.45 [4] Christopher J. Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” in K. Kroker, J. Keelan and P.M.H. Mazumdar (eds.) Crafting Immunity: Working Histories of Clinical Immunology (Hampshire, UK: Ashgate, 2008), p. 276-78 [5] F. Adams, “The Epidemic of Virulent Smallpox in Windsor and The Vicinity: February 1924,” Canadian Medical Association Journal 14 (8) (Aug. 1924): 692-96, article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1707573/; J.W. McIntosh, “The Vancouver Outbreak of Haemorrhagic Smallpox,” Canadian Public Health Journal 24 (3) (March 1933): 105-22 [6] Barreto and Rutty, “The Speckled Monster: Canada, Smallpox and its Eradication,” p. I-7; “Control Measures in British Columbia,” Canadian Journal of Public Health 38 (5) (May 1947): 218-19 [7] Barreto and Rutty, “The Speckled Monster: Canada, Smallpox and its Eradication,” p. I-7-8. [8] Armand Frappier Museum, “The Beginnings of the Institute at Laval,” 2008, http://www.virtualmuseum.ca/edu/ViewLoitLo.do?method=preview&lang=EN&id=18263 [9] Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” p. 280-81 [10] Ibid., p. 281; Katherine Dedyna, “Smallpox’s Big Man,” Victoria Times Colonist (May 21, 2010) [11] Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” p. 281-83; Paul Fenje, “Advances in Rabies Research,” Canadian Journal of Public Health 59 (6) (June 1968): 217-28 [12] D.A. Henderson, “The Saga of Smallpox Eradication: An End and A Beginning,” Canadian Journal of Public Health 70 (Jan-Feb 1979): 22. [13] Eric Jarvis, “A Contagious Journey Within a Culture of Complacency: The Smallpox Scare of 1962 in New York and Toronto,” Canadian Bulletin of Medical History 24 (Fall 2007): 343-66; available at: https://www.utpjournals.press/doi/abs/10.3138/cbmh.24.2.343 [14] Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” p. 282 [15] Connaught Medical Research Laboratories, Human Antigens Committee, February 21, 1963, Sanofi Pasteur Canada Archives, 83-005-16 [16] Barreto and Rutty, “The Speckled Monster: Canada, Smallpox and its Eradication,” p. I-10. [17] Ibid, p. I-11 [18] Steven Palmer and Gilberto Hochman, “A Canada-Brazil Network in the Global Eradication of Smallpox,” Canadian Journal of Public Health 101 (2) (March-Apr. 2010): 113-18; Steven Palmer, Gilberto Hochman, Danieli Arbex, “Smallpox eradication, laboratory visits, and a touch of tourism: travel notes of a Canadian Scientist in Brazil,” História, Ciências, Saúde – Manguinhos 17 (3) (July-Sept. 2010): 777-90, available at: https://www.redalyc.org/articulo.oa?id=386138050012 [19] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p. I-11; quoting private communication between D.A. Henderson and the Author, May 1, 2000. [20] Available at: https://apps.who.int/iris/handle/10665/67966 [21] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p., p. I-12; quoting letter, R.J. Wilson to D.A. Henderson, Sept. 19, 1968, Sanofi Pasteur Canada Archives, 88-001-11. [22] F. Fenner, D.A. Henderson, I. Arita, Z. Jezek, I.D. Ladny., Smallpox and its Eradication (Geneva: World Health Organization, 1988), p. 547-48; available here: http://apps.who.int/iris/handle/10665/39485 [23] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p. p. I-13; quoting letter, R.J. Wilson to D.A. Henderson, May 28, 1970, Sanofi Pasteur Canada Archives, 88-001-11. [24] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p. p. I-13, quoting letter, R.J. Wilson to D.A. Henderson, June 17, 1970, Sanofi Pasteur Canada Archives, 88-001-11. [25] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p. p. I-13, quoting letter, P. Fenje to D.A. Henderson, February 26, 1971, Sanofi Pasteur Canada Archives, 88-001-11, file 3/6. [26] Barreto and Rutty, "The Speckled Monster: Canada, Smallpox and its Eradication," p. p. I-13, quoting letter, D.A. Henderson to P. Fenje, March 3, 1971, Sanofi Pasteur Canada Archives, 88-001-11, file 3/6. [27] Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” p. 292-93; interview by author with Paul Fenje, October 2003. [28] John Donnelly, “Polio: A Fight in a Lawless Land: Targeted by Warlords but Armed with a New Vaccine, Teams Fan Across Somalia to Wipe Out the Disease,” Boston Globe, February 26, 2006, http://archive.boston.com/yourlife/health/diseases/articles/2006/02/27/polio_a_fight_in_a_lawless_land/; Ali Maow Maalin was interviewed while he helped with polio immunizations in Somalia during the WHO’s poliomyelitis eradication program. He died in 2013; https://en.wikipedia.org/wiki/Ali_Maow_Maalin
