[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
During the late 19th and early 20th centuries, diphtheria and pertussis (whooping cough) were major and growing public health threats in Canada and globally, especially among children. Both diseases are caused by specific bacterium types, which can spread between people and infect the body, mostly via the respiratory tract. Tetanus is also caused by a bacterium, but is not communicable and most often contracted through exposure in the environment, such as stepping on a rusty nail. As discussed in Article #4 of this series, Connaught Laboratories played a pioneering role in developing and producing diphtheria vaccine (toxoid) and proving its safety and remarkable preventive power. By the early 1930s, diphtheria was brought under control in Canada, but only if the toxoid was universally used. Nevertheless, research continued at Connaught to improve and refine diphtheria toxoid, focused primarily on preparing it on a larger scale.
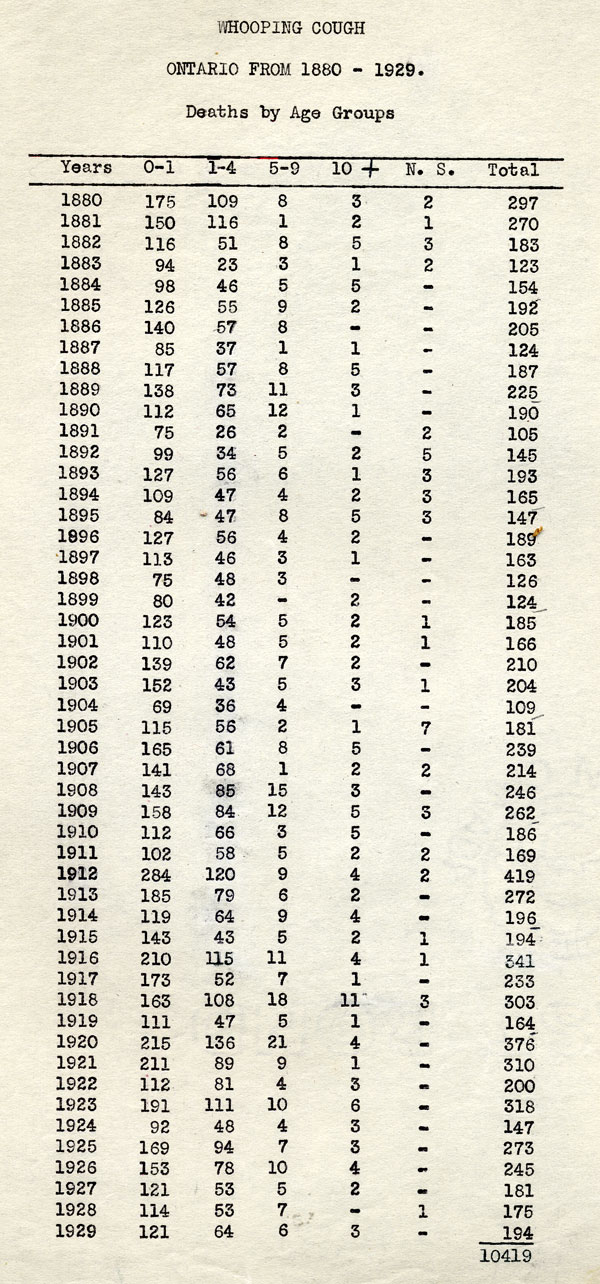
The fight against pertussis, however, would prove much more complex and challenging. A stubborn and highly infectious disease, pertussis is caused by the Bordetella pertussis bacterium and affects people of all ages, but is most debilitating and deadly among infants. Pertussis is more generally known as “whooping cough” because of the distinctive deep “whoop” sound it causes, especially in young children. Pertussis begins much like other respiratory infections, but soon the most serious stage of the disease starts, marked by persistent coughing fits that can last for several minutes; the severity and frequency of coughing can lead to vomiting, fainting, or seizures due to lack of oxygen. At the turn of the 20th century, pertussis killed 5 out of every 1000 children before their fifth birthday, mostly infants younger than one. During the late 1930s, pertussis case incidence rates in Canada hovered around 156 per 100,000 in most years, reaching a peak of some 20,000 cases in 1940; Ontario’s worst pertussis year was 1936 with 7,890 cases and 112 deaths recorded.
[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]
In 1906, Jules Bordet and Octave Gengou of the Pasteur Institute in Brussels identified the Bordetella pertussis bacterium as the cause of the disease. Gengou developed the first pertussis vaccine in 1912 using killed B. pertussis bacteria and in 1919, Connaught began producing this type of vaccine based on a mixture of standard strains of B. pertussis, although its effectiveness was quite limited. By the mid-1920s, it was discovered by Thorvald Madsen in Denmark and also Louis Sauer in Chicago, that preparing vaccine from B. pertussis freshly collected from the sputum of active cases was more effective. However, a more precise understanding of the importance of using fresh strains did not emerge until 1931 when the natural life cycle of B. pertussis was clearly defined. That year, P.H. Leslie and A. D. Gardiner in Britain demonstrated the existence four distinct phases in the life cycle of B. pertussis, the most significant of which for the production of an effective vaccine was “Phase I.”
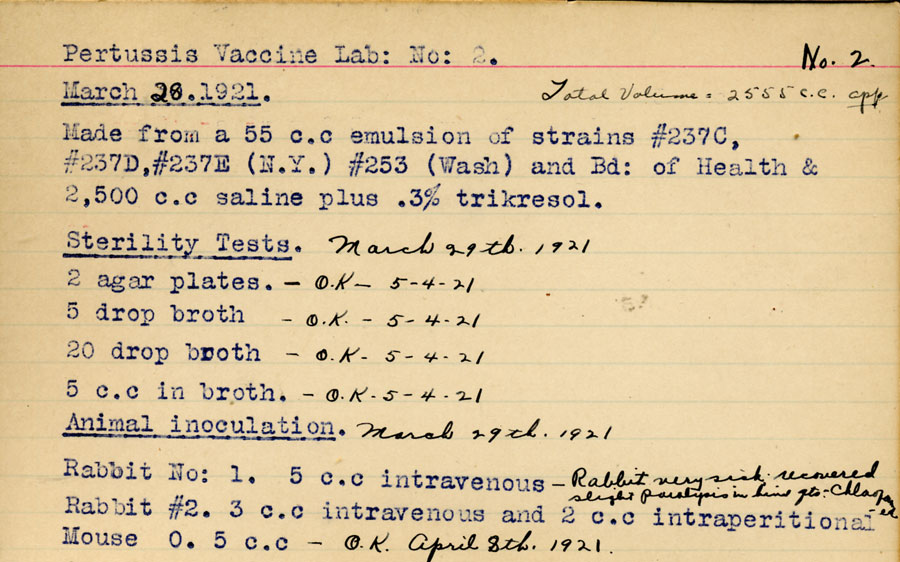
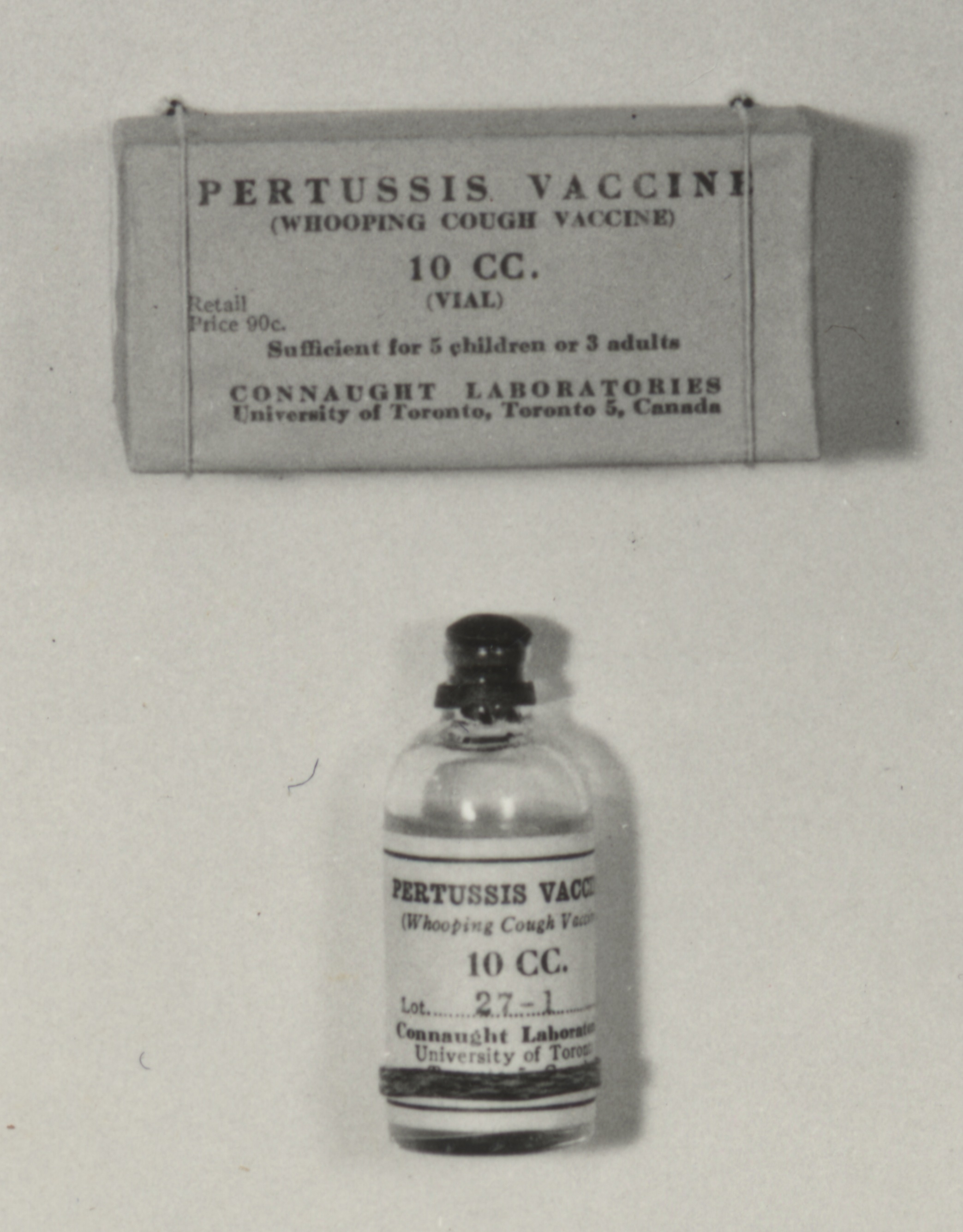
Starting in 1932, and building on the work of Madsen, Sauer and Leslie/Gardner, scientists at Connaught, led by Dr. Nelles Silverthorne, who was also a Resident Physician at the Hospital for Sick Children in Toronto, began work on preparing a more effective pertussis vaccine.[1] Based at the “Whooping Cough Clinic” he directed at HSC, Silverthorne focused on closely differentiating freshly collected phase I strains of B. pertussis from the standard stock strains that were being used in the current vaccine and saw “striking dissimilarities” between them.[2] In 1937, and based on encouraging results from several small vaccine trials in the Toronto area, coupled with localized trials of similar vaccines in the U.S. which also seemed to demonstrate protection, Connaught began limited distribution of a “fresh strain” pertussis vaccine. Supplies of Connaught’s vaccine, however, remained limited due to a laborious production process that relied on cultivating the slow-growing B. pertussis bacterium in small bottles on a solid nutrient medium.

Dr. Nelles Silverthorne (1901-2001), a pediatrician based at the Hospital for Sick Children in Toronto for 50 years, was born in Brantford, ON, and graduated in medicine from the University of Toronto in 1926. He specialized in infectious diseases, which in addition to pertussis, included polio and meningitis. He was also a pioneer of the oxygen tent and one of the first physicians in Canada to use penicillin.
[Sanofi Pasteur Canada Archives]

[Canadian Medical Association Journal 41 (1939): 263-65]
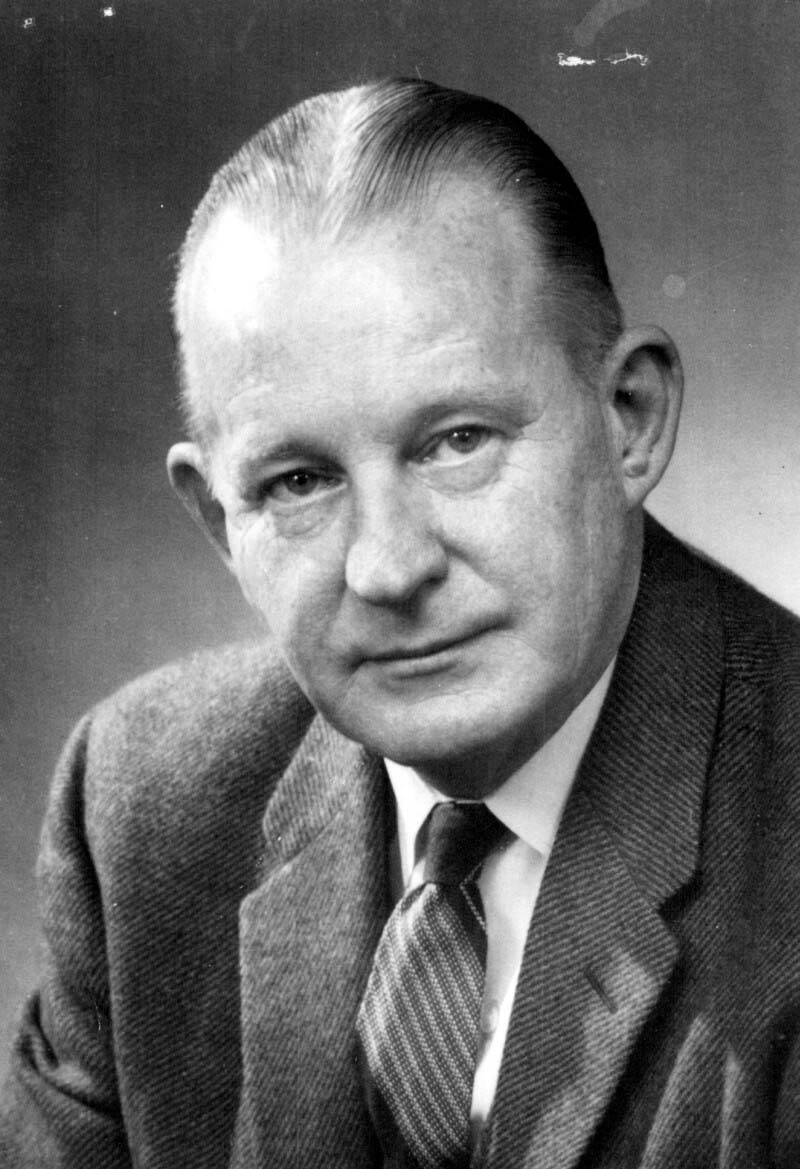
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Frustrated by the limits of the traditional solid media for growing phase-1 B. pertussis, especially the need to add blood or serum to stimulate growth, Dr. J.W. Hornibrook, of the United States Public Health Service, developed a “semi-synthetic” liquid medium. In 1939, Hornibrook first published about the new liquid medium, which was simpler in composition and based on a hydrolyzed casein (milk protein) solution as a primary source of amino acids.[3] At the same time, there was growing demand for Connaught’s pertussis vaccine from provincial governments and Hornibrook’s medium stimulated new research into larger scale production methods. During the early 1940s, and with a research team led by Dr. Robert J. Wilson, Connaught was one of the first vaccine producers to focus on modifying Hornibrook’s medium to optimize pertussis vaccine production.[4]
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Drs. Leone N. Farrell and Edith M. Taylor soon joined Wilson to help to tackle some of the outstanding difficulties in large-scale pertussis vaccine production based on a liquid medium, such as the type of bottles to use, optimum cultivation times, and how to aerate the cultures to promote bacterial growth. Farrell had previously solved a similar set of problems for the production of staphylococcal toxin by gently agitating culture bottles on a rocking machine and thought such a method could also work for cultivating B. pertussis. While Wilson focused on modifying the medium, Farrell worked with Taylor to work out a method to prepare pertussis vaccine on a larger scale that utilized rocked culture bottles.[5] This method enabled much more efficient production of larger amounts of pertussis cultures at a much lower cost, thus allowing provincial health departments to distribute the vaccine through local health departments and physicians at no charge to parents.
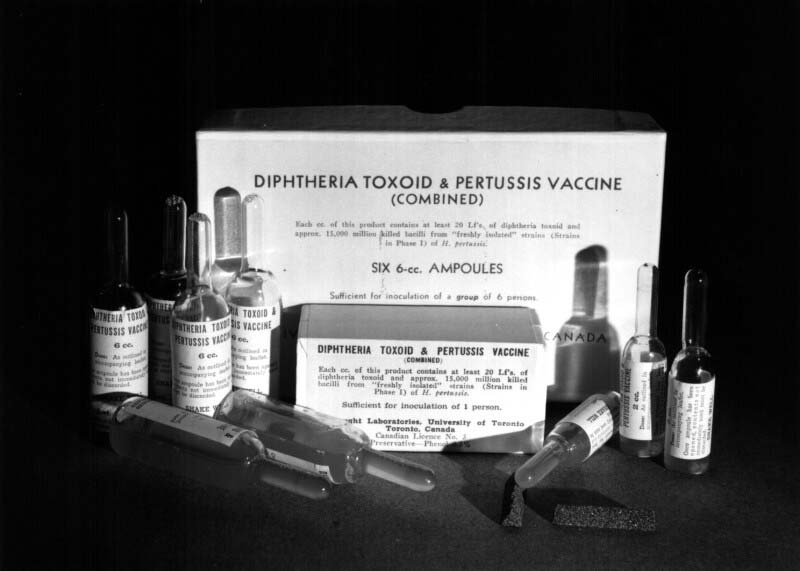
The improvements in pertussis vaccine production methods and efficiencies enabled pioneering work at Connaught focused on combining it with diphtheria toxoid to expedite protection against both diseases in a single shot. Diphtheria Toxoid-Pertussis vaccine (DP) was thus licensed in Canada in 1943, followed in 1947 by DPT, which included tetanus toxoid to also prevent tetanus in the same single shot. With the introduction of the combined vaccines, pertussis incidence and deaths fell sharply.[6]
The broadening use of pertussis vaccine in Canada and the United States after World War II, primarily in the DPT combination, coincided with the expansion of regulatory infrastructures within the U.S. government for the licensing of health products. With respect to biological health products, such as vaccines, the U.S. National Institutes of Health (NIH) established new production and testing standards for pertussis vaccines in 1947. The NIH’s standards served as the reference for the Canada’s Laboratory of Hygiene of the Department of National Health and Welfare, which had played a similar regulatory role since 1921, although with quite limited facilities and staff until the mid-1950s. A more robust and independent Canadian biologics regulatory capacity was facilitated by the development, testing and introduction of Connaught’s Salk polio vaccine (as discussed in Article #7) and was consolidated with the opening of a new Laboratory of Hygiene building in 1958.[7]

[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]
The NIH standards for pertussis vaccine were set in response to considerable variability in methods, quality control, safety and effectiveness among U.S. producers. In the United Kingdom, there was a similar effort by the Medical Research Council (MRC) to regulate pertussis vaccines and especially to evaluate the effectiveness of vaccines produced by several producers. During 1946-48, a series of carefully designed placebo-controlled clinical trials of vaccine prepared by four U.K. and U.S. producers were conducted in three U.K. cities and involved children aged 6-to-18 months with no history of whooping cough or pertussis vaccination. In 1951, the MRC reported the results, concluding that all vaccines provided protection, although there was notable variability in their effectiveness.[8] The MRC pertussis vaccine trials set a new standard for the controlled evaluation of a vaccine, but underscored variability in production methods.[9] A significant outcome was closer international cooperation among vaccine producers to better standardize such methods. As a respected vaccine producer situated within the academic research environment of a university, Connaught was well placed to play an important leadership role in such cooperative initiatives to better standardize and improve pertussis vaccines and bacterial vaccines generally.
During the 1950s and especially into the 1960s, consistently meeting rising minimum standards for pertussis vaccine production, testing and efficacy fueled new research at Connaught. This work was closely linked to process improvement work on diphtheria and tetanus toxoid vaccines. High priority was placed on increasing vaccine purity and developing new methods to scale up production capacity and efficiency to meet increasing Canadian and especially international demand. During this period, international exports became of growing importance for Connaught, spearheaded by the Labs’ pioneering production of polio vaccines (see Article #8).
[Sanofi Pasteur Canada Archives]

[British Medical Journal 1 (1951), p. 1463]
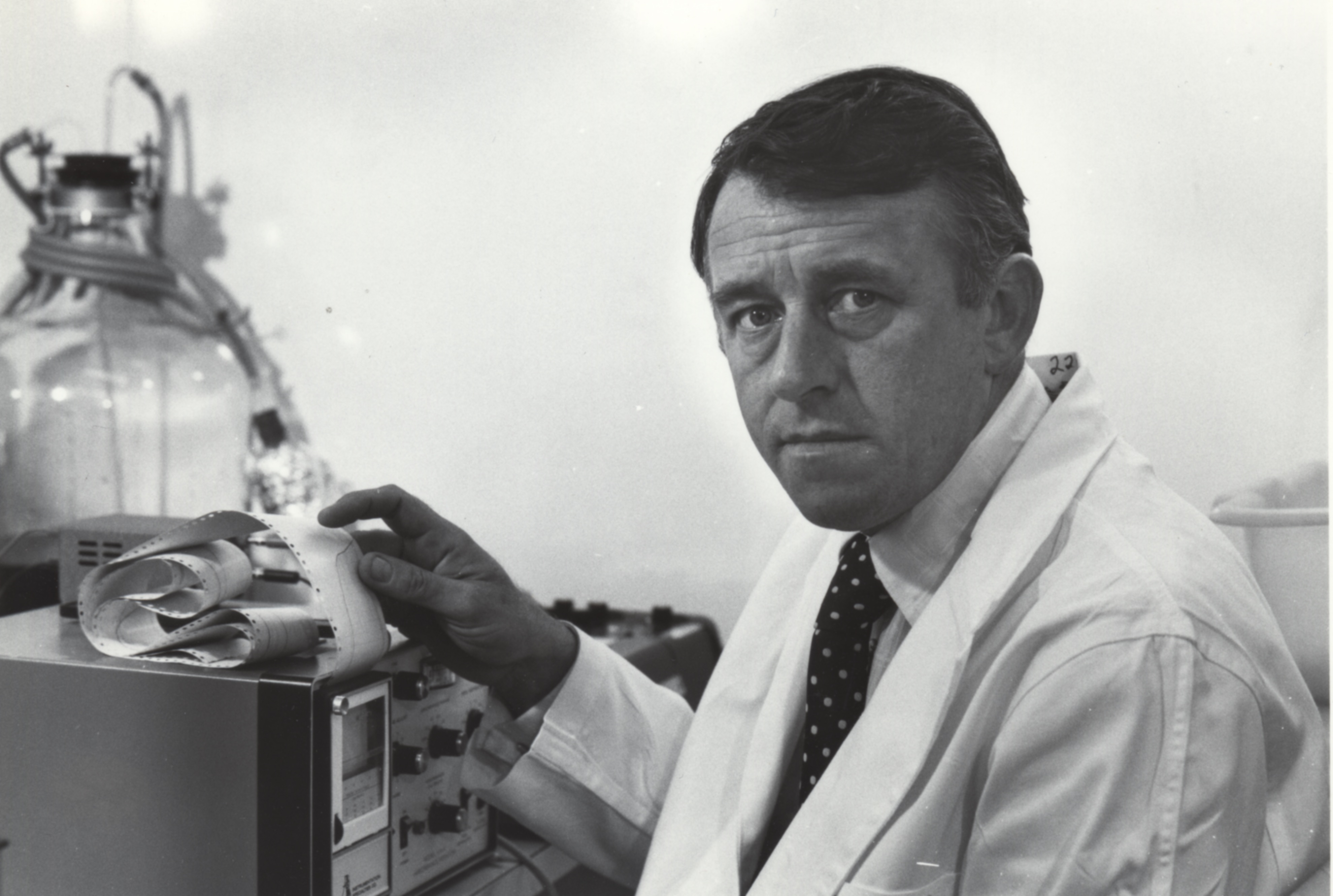
Central to the development of a new generation of bacterial vaccines at Connaught was Dr. Dennis W. Stainer. Originally from Liverpool, UK, Stainer received his Ph.D. in biochemistry from the University of Liverpool in 1957. The following year, he moved to Canada when he was awarded a National Research Council Post-Doctoral Fellowship at the Food and Drug Directorate in Ottawa. In 1960, Stainer joined Connaught, where he initially worked on the production of antitoxins. He soon shifted his focus to developing new methods and media to optimize the deep culture production of a new generation of highly purified bacterial vaccines, starting with diphtheria toxoid, which utilized large fermenters rather than bottles.
Stainer worked closely with Dr. James M. Corkill, who had joined Connaught in 1936. By the early 1960s, Corkill’s research focused on cultivating diphtheria and tetanus toxins in larger tanks, rather than small bottles, using variations of existing culture media. Such media were based on broths of veal or beef muscle. They also included casein hydrolysate, which, as noted earlier, is prepared from the milk protein known as casein. However, there was growing pressure from national regulators to reduce the presence of animal proteins in all vaccines due to potential allergic reactions. The first meat-free semi-synthetic medium for diphtheria toxoid production had been developed by Dr. J.H. Meuller of Harvard University in 1939 and was suitable for small batches prepared in stationary bottles.[10] However, when applied to larger-scale deep culture production, Meuller’s medium gave poor yields. While Stainer and Corkill worked on how best to prepare larger batches of diphtheria toxoid, it became clear that the goal needed to be to not just reduce the presence of animal proteins in vaccines, but to reduce such proteins to zero, especially in diphtheria toxoid.

[Sanofi Pasteur Canada Archives]
As Corkill and Stainer initially prepared larger experimental batches of diphtheria toxoid they found that the culture media needed to contain beef digest to maintain high yields. However, they also discovered convincing evidence of sensitizing or allergic reactions to such beef proteins in guinea pig tests. These reactions suggested the same type of reactions were possible in children who would receive the toxoid, the potential numbers of which would grow with larger production and international use. There had been other attempts elsewhere to prepare a synthetic media for larger scale diphtheria toxoid production, but they were impractical and too expensive for routine use.[11]

[Canadian Journal of Microbiology 13 (1967), p. 963]
Stainer worked with Corkill and Machiel J. Scholte to develop a new semi-synthetic diphtheria culture medium, which was based on “N.Z. Amine Type A,” a commercially available enzymatic digestion of the milk protein, casein. Key to the practical utility of the new medium was that it made possible more accurate control of iron, calcium and phosphate levels to optimize bacterial culture growth through precise additions of ferrous sulphate, calcium chloride and inorganic phosphate solutions. As their seminal 1968 paper emphasized, this new medium was inexpensive and easy to prepare and yielded high titres of diphtheria toxin.[12] This new medium quickly became an international standard for producers of highly purified diphtheria toxoid on a large scale.[13]
The experience Stainer gained in developing the new medium for diphtheria toxoid production in large fermenters was applicable to similar work with pertussis vaccine. Indeed, during the late 1960s, the diphtheria and pertussis vaccine development work was part of a coordinated product improvement program at Connaught based on adapting production methods and media to new 140-litre fermenters to increase vaccine volumes and purity, and reduce costs.[14]

[Canadian Journal of Public Health 54 (Nov. 1963), p. 518]
Thus, at about the same time as Stainer was finalizing the new diphtheria medium, he was also working on modifications to B. pertussis culture media to enable larger-scale production. Since Hornibrook developed the first liquid medium for culturing B. pertussis in 1939, many scientists had experimented with modifications, including R.J. Wilson at Connaught in the mid-1940s, as discussed earlier. These mixtures were based on casein hydrolysate, to which various salts, growth factors and either starch, charcoal, or anionic resins were added. In 1963, Wilson prepared a more precisely defined synthetic media, which included a liver co-enzyme mixture.[15] While Wilson’s medium yielded good growth of phase 1 B. pertussis organisms, it proved too complex and ultimately unsuitable for large-scale vaccine production.
Stainer worked again with Scholte to modify and simplify Wilson’s medium. The key ingredients were sodium glutamate and proline, the concentrations of which affected the yields of B. pertussis. This new starch-free medium was inexpensive and easy to prepare in large volumes and produced a vaccine that was much more immunogenic than the existing product and remained stable for prolonged periods at high temperatures. As Stainer concluded in a report to Connaught’s Human Antigens Committee in February 1969, “this medium had very interesting possibilities both from a practical and academic standpoint.”[16] The medium improved vaccine production capacity and purity, while also providing a more precise means to study B. pertussis itself. First presented at an International Symposium on Pertussis in 1969, and then published widely in a 1970 paper, the new medium became known as the “Stainer-Scholte” medium.[17] It would soon become the standard media for B. pertussis cultivation and for large-scale pertussis vaccine production internationally.[18]
Connaught’s pertussis and diphtheria vaccines improvement work during the 1960s was primarily conducted in converted stables (Buildings #14 and #15) originally built in 1928. While some work was accommodated in other small buildings, there was clear need to consolidate Connaught’s bacterial vaccines department in a new building. There was a pressing need for more space and upgraded facilities to meet growing production demands, especially internationally. There was also clear need to meet increasingly strict Canadian and U.S. regulatory demands which included more precisely defined standards for vaccine production facilities.
Initial plans to upgrade the Bacterials Department began in 1956, but formal approval from the University of Toronto Connaught Committee, which oversaw the Labs, to proceed with planning a new building, was not given until 1969. By this time, it was clear that without new facilities, Canadian and U.S. regulators would no longer permit Connaught to prepare and distribute bacterial vaccines. However, the complex process of developing detailed plans that met regulator’s stringent standards took until 1971 before they were accepted.[19]

[Sanofi Pasteur Canada Archives]

[Journal of General Microbiology 63 (Oct. 1970), p. 211 (there was an error in the date in the journal header that indicated a 1971 publication date, but it was actually 1970)]

[Journal of General Microbiology 1968, 14(10): p. 1155]

[Sanofi Pasteur Canada Archives]
To further complicate matters, the University of Toronto was unable to fund the new building project. The Labs would have to finance the estimated $1.5 million price of the new building itself, primarily from net earnings derived from export sales. Connaught’s earnings from Canadian sales were quite limited as its primary customers were provincial governments to which vaccines were sold on a cost basis. For international sales, Connaught competed with other vaccine producers and could earn a modest profit. However, future growth of export sales and earnings depended upon the new building. The new facility would be known as Building 90 and would provide 40,000 square feet (or 3,700 square meters) of floor space on the main floor and basement. There would be expanded production space for new cultural and protein purification methods and for the development, production and improvement of new bacterial vaccines. Construction began in December 1971 and Building 90 officially opened on June 1, 1973.
Persistent research and innovation focused on the improvement of biological health products, particularly bacterial vaccines to prevent diphtheria and pertussis, was central to the mission of Connaught Laboratories while it was a vital part of the University of Toronto. As we have seen, such persistence intensified during the late 1960s with the development of new large-scale production methods to meet growing Canadian and especially international demand, the key to which was pioneering innovations in preparing a new generation of synthetic culture media for diphtheria and pertussis vaccines that established new global standards. As will be examined in the next article of this series, from the late 1950s and through the 1960s, such research and innovative persistence was also focused on improving or refining several other critical biological health products – rabies vaccines, tuberculin and insulin - with similarly significant international impacts.

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Useful Resources:
Bator, Paul: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories Limited, Volume 2, 1955-1975; With An Update to the 1990s (Ottawa: Canadian Public Health Association, 1995) Defries, Robert D.: The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (Toronto: University of Toronto Press, 1968) “The History of Vaccines: An Educational Resource by the College of Physicians of Philadelphia;” https://www.historyofvaccines.org/ Plotkin, Stanley; Orenstein, Walter; Offit, Paul; Edwards, Kathryn M: Plotkin’s Vaccines (7th edition), (Elsevier, 2018). Rutty, Christopher J. and Sullivan, Susan: This is Public Health: A Canadian History (Canadian Public Health Association, 2010), online eBook: https://www.cpha.ca/history-e-book Sanofi Pasteur Canada, “The Legacy Project”: http://thelegacyproject.ca “Vaccines & Immunization: Epidemics, Prevention & Canadian Innovation:” The Online Exhibit, Museum of Health Care, Kingston (2013-14); http://www.museumofhealthcare.ca/explore/exhibits/vaccinations/ “Within Reach of Everyone: The Birth, Maturity & Renewal of Public Health at the University of Toronto,” Dalla Lana School of Public Health website feature: http://www.dlsph.utoronto.ca/history/
Endnotes:
[1] Crawford S. Anglin, “Remembrances of an old infectious diseases doctor,” Pediatrics and Child Health 5 (3) (April 2000): 148-49; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2817770/ [2] Nelles Silverthorne and Donald T. Fraser, “The Bacteriological Diagnosis of Whooping-Cough,” Canadian Medical Association Journal 32 (4) (April 1935), p. 368; full article at: https://www.ncbi.nlm.nih.gov/pubmed/20319835 [3] J.W. Hornibrook, “Cultivation of Phase I H. Pertussis in a Semisynthetic Liquid Medium,” Public Health Reports 54 (41) (Oct. 13, 1939): 1847-51 [4] R.J. Wilson, “The Production of Phase I Pertussis Vaccine in Casein Hydrolysate Broth,” Canadian Journal of Public Health, 36 (Aug. 1945): 321-26. [5] Leone Farrell and Edith M. Taylor, “Notes on the Production of Phase I Pertussis Vaccine in Fluid Medium,” Canadian Journal of Public Health, 36 (Aug. 1945): 326-27. [6] R.J. Wilson, “Canada’s Experience with Multiple Antigens,” Canadian Journal of Public Health 53 (11) (Nov. 1962): 457-62. [7] “A New Laboratory of Hygiene, Ottawa,” Canadian Journal of Public Health, 49 (Sept. 1958): 402-04. [8] “The Prevention of Whooping-Cough By Vaccination: A Medical Research Council Investigation,” British Medical Journal, 1 (June 30, 1951): 1463-71; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2069253/ [9] T. Jefferson, “Why the MRC Randomized Trials of Whooping Cough (Pertussis) Vaccines Remain Important More Than Half a Century After They Were Done,” Journal of the Royal Society of Medicine 100 (July 2007): 343-45; article available at: https://journals.sagepub.com/doi/full/10.1177/014107680710000720 [10] J. Howard Mueller, “A Simplified Formula for Diphtheric Toxin Broth,” The Journal of Immunology, 37 (Aug. 1, 1939): 103-12. Mueller conducted part of his research at the Faculty of Medicine at Dalhousie University, as well as at the Nova Scotia Provincial Laboratory, in Halifax. The full article is available at: http://www.jimmunol.org/content/37/2/103 [11] D.W. Stainer, “Preparation and Properties of Diphtheria Toxoids in Submerged Culture: 1. Presence of Bovine Antigens,” Canadian Journal of Microbiology, 13 (8) (1967): 963-68; article available at: http://www.nrcresearchpress.com/doi/abs/10.1139/m67-129#.XC4yIs9KiRu [12] D.W. Stainer, J.M. Corkill and M.J. Scholte, “Preparation and Properties of Diphtheria Toxoids in Submerged Culture: III. Development of a New Semisynthetic Medium,” Canadian Journal of Microbiology, 15 (10) (1968): 1155-60; article available at: http://www.nrcresearchpress.com/doi/abs/10.1139/m68-193#.XC5Ygc9KiRt [13] Audrey I. Tchorbanov, Jordan D. Dimitrov, Tchavdar L. Vassilev, “Molecular composition of diphtheria toxoid produced using semi-synthetic and meat extract-based broths,” World Journal of Microbiology and Biotechnology 20 (2004): 211-17 [14] Human Antigens Committee, Connaught Medical Research Laboratories, Feb. 7, 1967; Feb. 6, 1968, Sanofi Pasteur Canada Archives, 83-005-16. [15] R.J. Wilson, “Cultivation of Bordetalla pertussis (Phase I) in a Chemically Defined Medium,” Canadian Journal o Public Health, 54 (Nov. 1963): 518-23. [16] Human Antigens Committee, Connaught Medical Research Laboratories, Feb. 10, 1969, Sanofi Pasteur Canada Archives, 83-005-16. [17] D.W. Stainer and M.J. Scholte, “A Simple Chemically Defined Medium for the Production of Phase I Bordetella pertussis,” Journal of General Microbiology, 63 (1970): 211-20; article available at: https://mic.microbiologyresearch.org/content/journal/micro/10.1099/00221287-63-2-211 [18] See, for example: Carl Heinz Wirsing von Koenig, Angelika Taken and Horst Finger, “Use of Supplemented Stainer-Scholte Broth for the Isolation of Bordetella pertussis from Clinical Material,” Journal of Clinical Microbiology 26 (12) (Dec. 1988): 2558-60; article available at:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC266945/ [19] J.K.W. Ferguson, “A New Building for Bacterial Vaccines and Toxoids,” Contox (Connaught Medical Research Laboratories Newsletter) #41 (Nov. 1971): 1-2; article available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-10/CONTOX-1971-11-n41-B90.pdf
