
[Sanofi Pasteur Canada Archives]
Paul Fenje & Finding a New Rabies Vaccine

[Sanofi Pasteur Canada Archives ]
During 1958-59, Ontario endured a major rabies outbreak, bringing incidence levels among cattle and domestic dogs and cats, as well as humans, to a new peak. The outbreak’s origin could be traced back a decade to the North West Territories, from where it slowly spread towards the southeast, intensifying in southern Ontario in 1959. Wild foxes were the main virus reservoir, with their bites directly and indirectly infecting livestock, pets, and people.[1] Indeed, two rabid wild foxes were discovered on Connaught’s Dufferin Division property, their arrival coinciding with a growing rabies research program led by Dr. Paul Fenje. As recounted in Article #9 of this series, Fenje joined Connaught in 1958 after escaping from Yugoslavia’s Communist rule. He provided exactly the specialized virology skills needed to respond to the growing rabies crisis.[2]
Rabies is an acute, progressive viral disease of the central nervous system, transmitted from animal to animal, or animal to human, via exposure to infected saliva. Derived from the Latin word for “madness,” and characterized by hydrophobia, or “fear of water,” rabies is most often spread through animal bites and is virtually 100% fatal unless treated with the rabies vaccine, which was first developed by Louis Pasteur in 1885. Although effective, Pasteur’s vaccine had inherent risks: prepared from rabies-infected brain or spinal cord tissue, usually from rabbits, the vaccine included animal nerve tissue that could cause significant neurological damage to the patient.
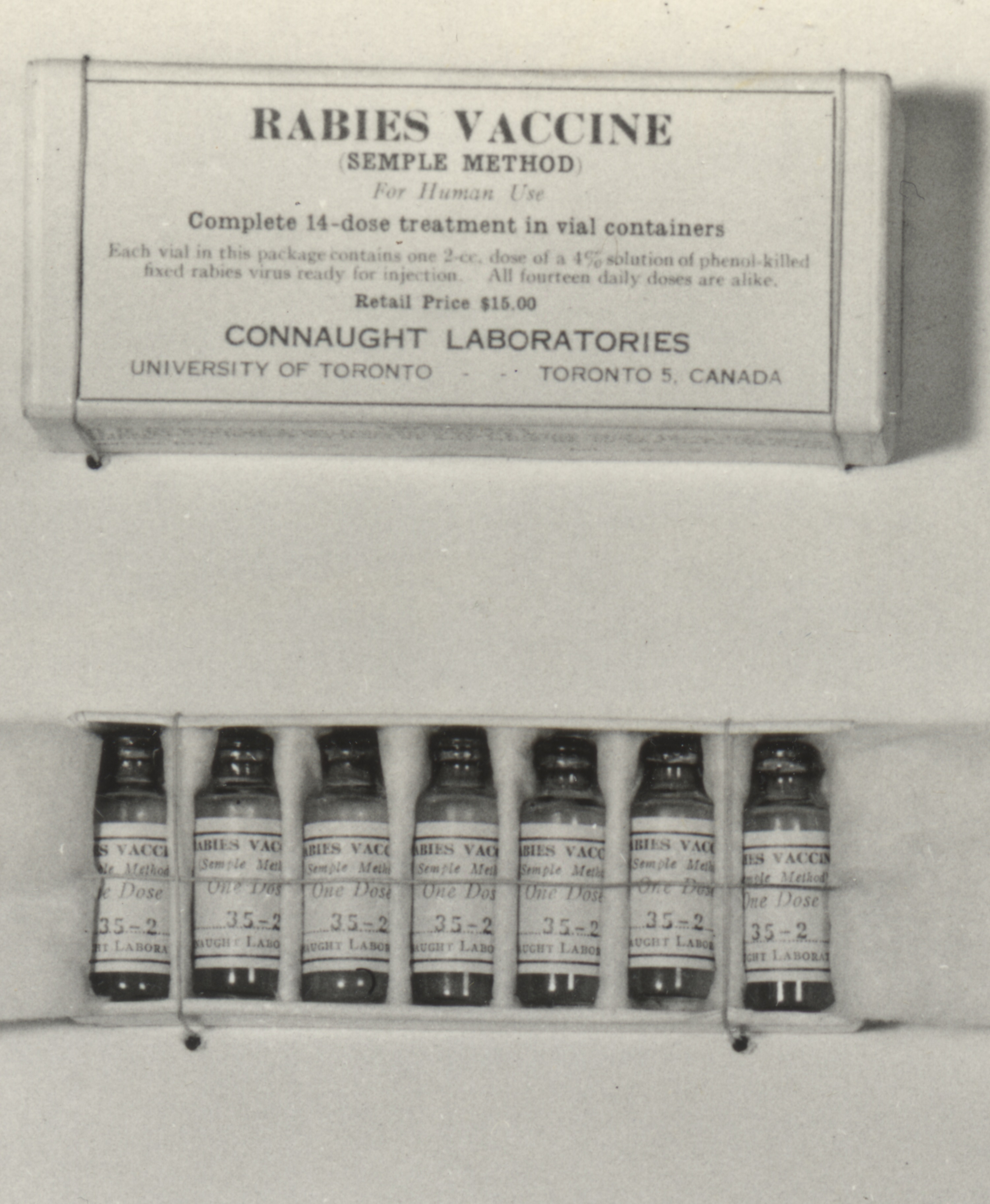
Connaught Laboratories began preparing the Pasteur-type rabies vaccine in 1914, and then a modified “Semple” type starting in the late 1920s. Originally developed by David Semple in 1911, the “Semple” vaccine was prepared from the rabies infected brain tissue of sheep, which was then inactivated with phenol. The Semple vaccine was as effective as the Pasteur type, but similarly risky, although it could be prepared on a larger scale and more easily packaged, preserved and distributed. During the 1958-59 Ontario outbreak, some 3,000 people bitten by, or otherwise exposed to suspected rabid animals, were given the Semple rabies vaccine. The risks of the vaccine had to be weighed against the almost certain fatal outcome of a rabid animal bite. The risk of a severe neuroparalytic accident after a full course of the vaccine was about 1 in 1,000, although most recovered. Fortunately, there were no serious reactions to the vaccine reported among those who received it.[3] During the Ontario rabies outbreak, there was only 1 human death; a young boy in Port Perry succumbed in 1958 after being bitten by a rabid skunk, but he did not receive vaccine treatment.[4]

[Globe & Mail, Jan 8, 1959, p. 9]
[Sanofi Pasteur Canada Archives]

[Wikimedia Commons]
The Semple vaccine also proved effective in preventing rabies in livestock, with Connaught often called upon by the federal government to supply the vaccine to control rabies outbreaks among livestock.[5] In the late 1940s, Hilary Koprowski and Herald Cox at Lederle Laboratories in the U.S. developed a new preventive rabies vaccine for livestock and dogs; it was based on the Flury strain of rabies virus that was weakened or attenuated and grown in chicken eggs. However, this type of vaccine was not safe for humans. The Flury virus strain did not readily replicate in humans and the animal protein content in the vaccine was too high for human use.[6]
[Sanofi Pasteur Canada Archives ]

[Sanofi Pasteur Canada Archives]
In 1958, soon after arriving at Connaught Laboratories, Paul Fenje found himself facing the limits of scientific understanding about almost all aspects of rabies, the limits of the rabies vaccine, and quite fearful of the vaccine’s inherent dangers as rabies incidence increased among animals and its human use increased. His fears and frustrations about the state of knowledge about rabies stood in sharp contrast to the rapid progress against other virus diseases, especially polio. However, rabies was a very different type of virus: very slow, very elusive and very deadly, and so far very resistance to close study. Fenje was very determined to overcome such limits. As he emphasized in a July 1959 conference presentation, “The situation will not improve until research has succeeded in producing a potent vaccine free from brain tissue.”[7] The key to such a vaccine was figuring out how to cultivate the rabies virus in non-nervous cell tissue cultures.
However, progress in cultivating rabies virus in non-nervous tissue culture had remained very slow until 1958 when R.E. Kissling, of the U.S. Department of Health Laboratory in Alabama, reported significant results by using hamster kidney tissue. However, the amount of virus he was able to produce and its infectivity was quite limited.[8] Fenje built on Kissling’s work, but used a different virus strain and a modified cell cultivation method based on a special culture tube that incorporated a dialyzing membrane. And as reported in his seminal 1960 article, Fenje was able to maintain the continuous culture of the hamster kidney cells for many weeks, thus making possible the cultivation of virus culture fluids of high infectivity.[9]
[Sanofi Pasteur Canada Archives]

[Canadian Journal of Microbiology 6 (6) (Dec 1960): 605-09]
Once the rabies virus had been established in tissue culture, Fenje expected further progress along two lines of investigation: 1) an increase in general knowledge of the biological properties of the rabies virus, and 2) significant improvement in anti-rabies vaccines used for human and animal prophylaxis. In a follow-up article, Fenje reported significant progress in the second line of research, demonstrating a further refined method of virus cultivation and the preparation of an effective experimental vaccine based on virus inactivation using formaldehyde. However, human testing would not be possible until a serum-free synthetic culture medium was developed.[10] Fenje’s work also facilitated significant progress along the first line of investigation, essentially bringing the rabies virus out of hiding to allow it to be seen for the first time.
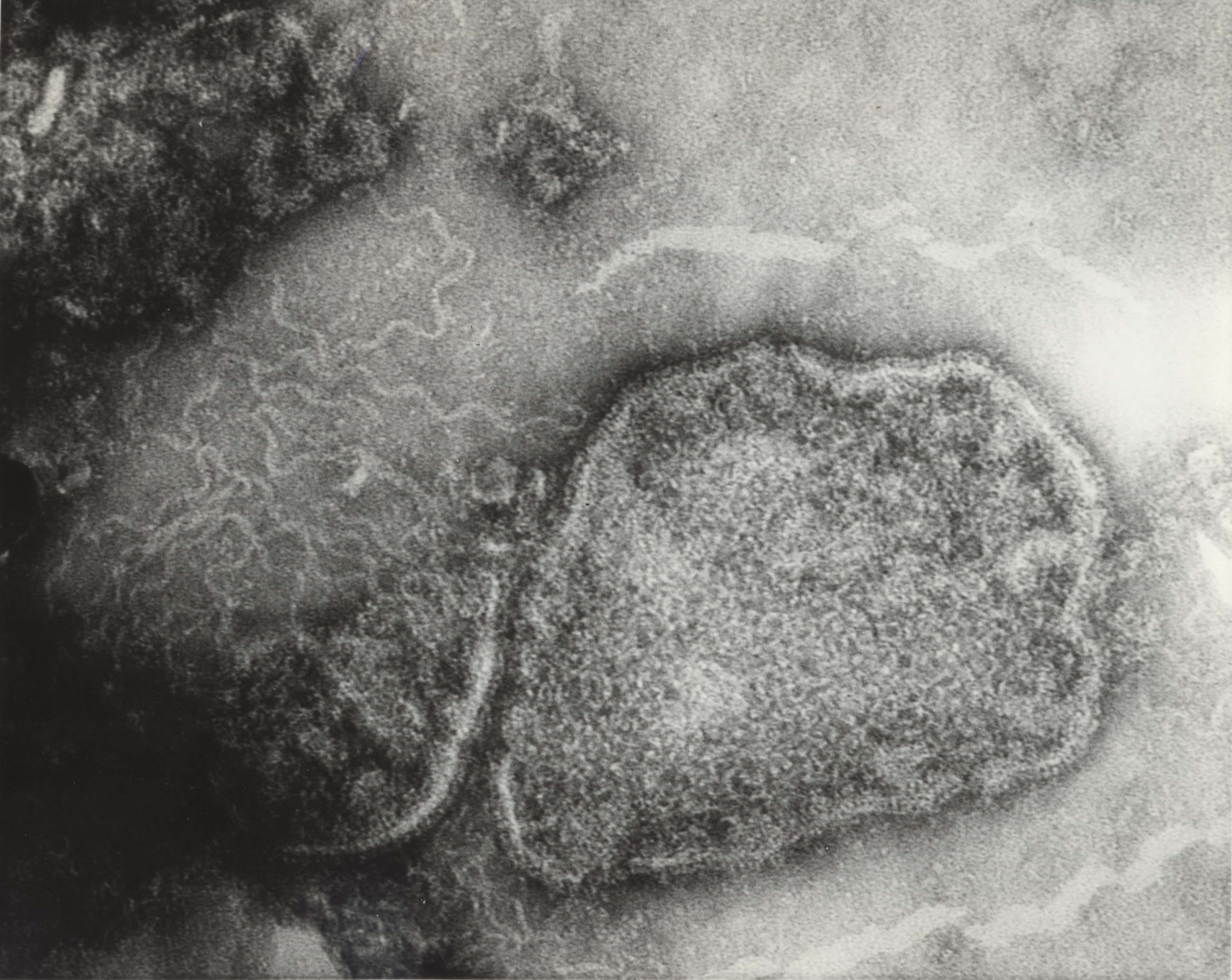
Although estimates had been made about the size and structure of the rabies virus, its unstable nature and the way it infected nerve tissue obscured even the power of the electron microscope to get a clear view. The University of Toronto pioneered the development of the electron microscope and Connaught acquired its own in the late 1940s to support an expanded virus research program focused mainly on poliovirus studies.[11] Fenje directed new electron microscope attention on the rabies virus in 1962 with the assistance of Les Pinteric, who like Fenje, originally came from Serbia. Pinteric’s thorough knowledge of electronic engineering impressed Connaught’s Director, who hired him to take charge of the Labs’ electron microscope.[12] Fenje and Pinteric also worked with scientists from the Ontario Cancer Institute, Dr. A.F. Howatson and J.D. Almeida, to take what would be the first clear photographs of the rabies virus at 280,000 times magnification. A new method of negative staining helped to differentiate the virus from the surrounding tissue.[13]

[Globe & Mail, Jan. 28, 1966, p. 35]

[Sanofi Pasteur Canada Archives]
With the growing threat of animal rabies and inevitable human exposure, especially among veterinarians, lab personnel, dog catchers, forest conservation workers, and farmers, Fenje felt an urgency to further develop the inactivated tissue culture rabies vaccine and test it on volunteers drawn from these high-risk groups. A key step in human testing was the use of a culture media free of animal proteins to cultivate the rabies virus. However, vaccine prepared from a synthetic media resulted in a less potent vaccine than when prepared with culture media containing animal serum. Fenje soon found that use of an adjuvant to boost the magnitude and rapidity of the immune response yielded a safe and effective vaccine.[14] Initial trials of the pre-exposure vaccine proved successful enough to prompt a submission for a Canadian license in 1967. Approval was finally given in November 1969; this was the first license granted for a human tissue culture rabies vaccine in the world.[15]
While Fenje was focused on the human rabies vaccine, his method to cultivate rabies virus in tissue culture also made possible the development of a new veterinary vaccine for the prevention of rabies among a wide variety of animals. By 1964, a research team at Connaught led by Dr. Melvin K. Abelseth used rabies virus grown by Fenje in hamster kidney cells to develop a unique strain of the rabies virus that grew vigorously in pig kidney cells.[16] This novel “ERA” strain was named after the Connaught research team that developed it (“E” for Evelyn Gaynor and “R” for Alex Rockitnicki, both technicians, and “A” for Dr. Abelseth), and then used to prepare a new live virus rabies vaccine that was remarkably effective in domestic dogs. Existing vaccines only provided limited one-year immunity, but the ERA vaccine provided three years’ protection and also proved highly effective in cattle. In 1966, Connaught’s ERA vaccine was licensed in Canada and the U.S. and quickly adopted in many other countries, particularly where cattle rabies was a major problem.[17]
Silvio Landi & Improving Tuberculosis Testing
[Source]
While rabies was an increasing threat when Paul Fenje joined Connaught in 1958, tuberculosis (TB) appeared well controlled when Dr. Silvio Landi joined the Labs that same year. Landi’s initial studies in Italy were focused on botany and mycology before moving to Canada and earning his MA and Ph.D. in botany from the University of Toronto. His initial work at Connaught was with typhoid and cholera vaccine production and testing before shifting his focus almost exclusively to tuberculosis-related BCG vaccine and tuberculin research, development and production. Landi recognized that consistently accurate diagnostic testing for TB remained an unresolved challenge in Canada and globally.

[Photo courtesy of Dr. Landi’s niece, Marta Pesin Fernandes]
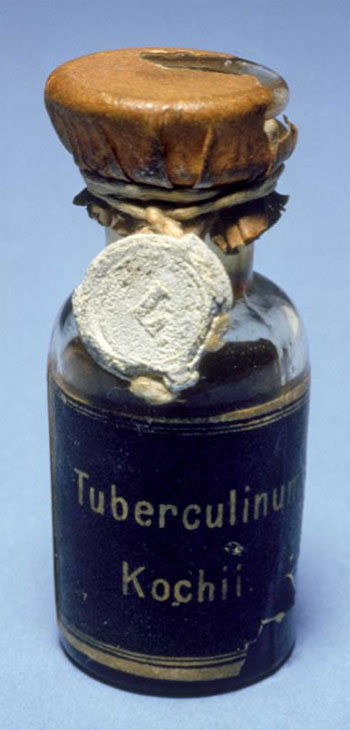
The tubercle bacterium was first cultivated in 1882 by Robert Koch, who confirmed it as the cause of tuberculosis. In 1890, Koch prepared “tuberculin,” which he described as a “brownish transparent fluid” culture filtrate of the tubercle bacilli that he initially thought could be used to prevent or even cure TB. It was soon apparent that tuberculin would neither prevent of cure tuberculosis, but “Koch’s Lymph” as it was also known, proved effective as a skin-active TB diagnostic agent. When a small amount of tuberculin was injected into the skin of a person, Koch observed that there was a marked difference in the sensitivity response if the person had been exposed to TB. Tuberculin was an active substance that could detect hypersensitivity to the tubercule bacillus or its components. As was later clarified, if a small, firm and swollen indurated spot developed at the site of injection within 24-48 hours, the reaction was positive and indicated the person had been exposed to the TB bacilli and may have the disease. If there was no reaction, the person was not infected.[18] Such tuberculin tests were also effective with animals, especially dairy cattle, to prevent the spread of TB through milk.
Tuberculin was a crude filtrate of Mycobacterium tuberculosis var. homonis that also contained various proteins and other by-products. By 1935, when Connaught began preparing and distributing tuberculin (known as “Old Tuberculin”), its production process had changed little since the time of Koch.[19] As an impure product, the original tuberculin’s testing sensitivity was limited and reactions to it could be problematic. However, a purified form of tuberculin, known as “tuberculin purified protein derivative,” or “PPD,” was developed by Florence B. Seibert, at the University of Pennsylvania, in 1934. A variation of Seibert’s PPD, called “PPD-S,” was then adopted as the U.S. reference standard in 1944, and internationally in 1952.[20] Connaught, however, continued to produce “Old Tuberculin,” but did not prepare PPD.
In 1958, when Landi arrived at Connaught, he saw an opportunity for the Labs to prepare a higher quality, more purified form of PPD to meet increased demand for such a product in expanding TB testing programs in Canada and in the United States. Landi focused on modifying and standardizing an older PPD production method that would be based on a single strain of M. tuberculosis instead of three, and would utilize a pure synthetic culture medium.[21] The single strain was known as the “Johnston” strain, originally isolated in 1920 from a patient in Toronto with pulmonary tuberculosis. Landi’s production method for “Connaught” Tuberculin PPD, as described in his seminal 1963 article, “Preparation, Purification, and Stability of Tuberculin,” quickly became recognized as the standard method, particularly when applied using the “Mantoux” TB testing procedure.[22] Several TB testing procedures had developed over time, including the cutaneous skin test (Pirquet), a patch test (percutaneous), and the most widely used Mantoux test, which involved applying tuberculin to the skin intracutaneously. There was also the Heaf test method, which involved a mechanical device. The various testing procedures required slight differences in the final PPD formulation. Connaught’s tuberculin PPD for the Heaf and Mantoux test method, were licensed in Canada in January 1960, and then in the United States in May 1960 (Heaf) and June 1966 (Mantoux).[23]

[Dominion Sanitary Journal, Aug. 1884, p. 311]
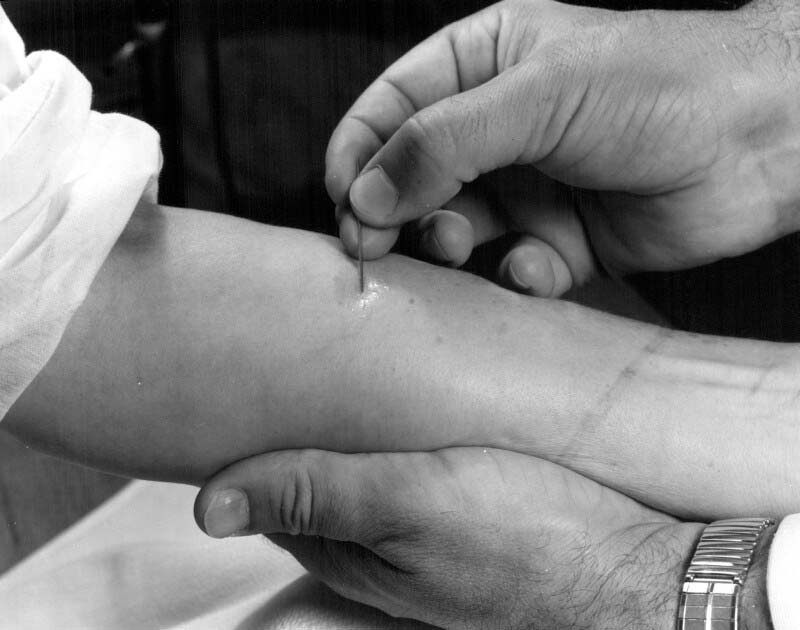
[Sanofi Pasteur Canada Archives]

[Applied Microbiology, 11 (5) (Sept. 1963): 408-12]
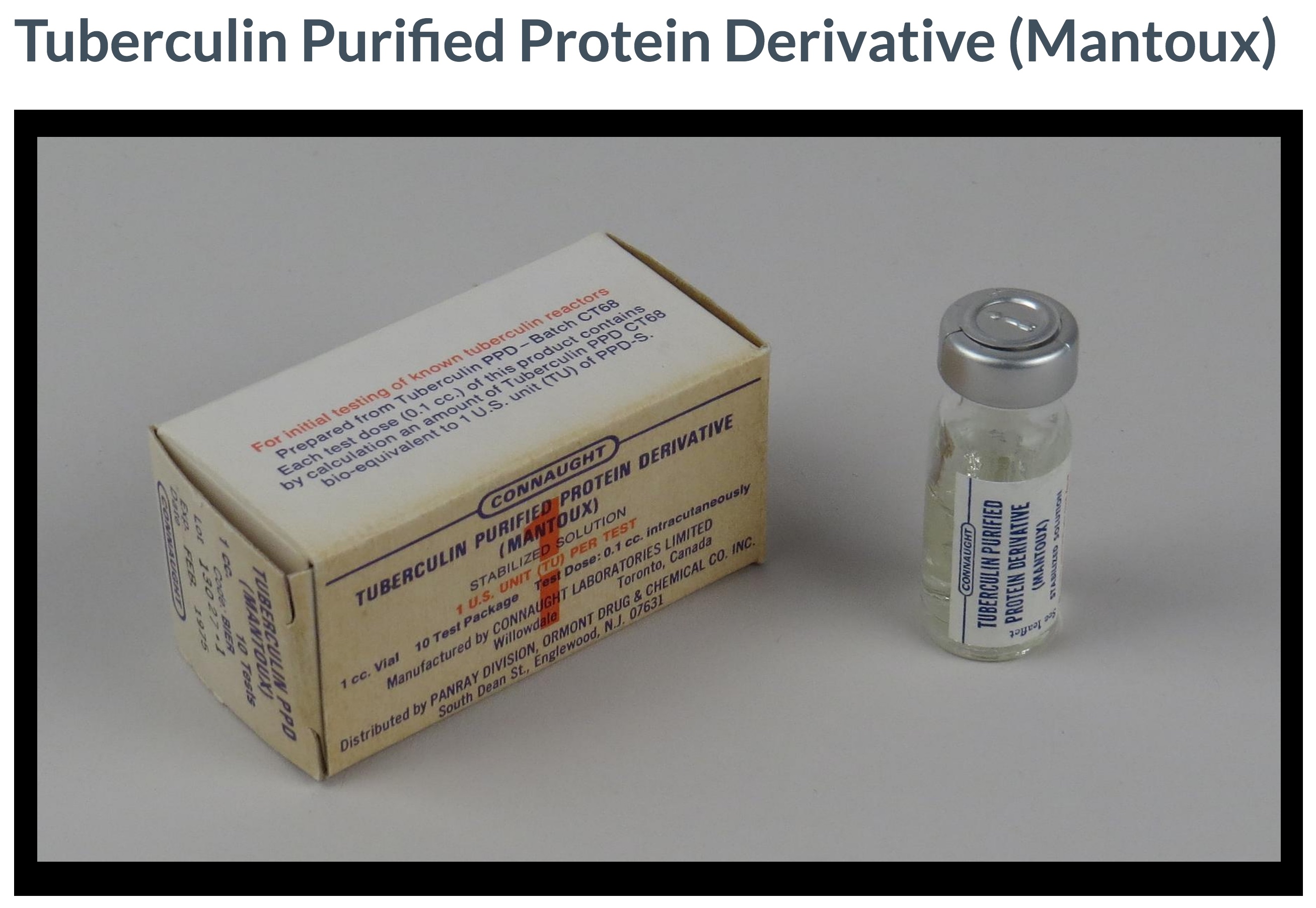
[Smithsonian National Museum of American History]

[Sanofi Pasteur Canada Archives]
While Landi’s PPD production method served as the standard, it soon became clear that when different labs prepared PPD, even very slight variations in materials, methods, and production conditions could result in variations in the final product that could lead to inaccurate TB skin testing results. At the same time, there was a growing international demand for PPD. Landi’s clear leadership in the field put Connaught in a key position to resolve both challenges at the same time. In April 1968, Connaught’s Director J.K.W. Ferguson asked Landi to prepare a “master lot” of dried PPD. Landi agreed to prepare 400 grams by combining ten completed lots of concentrated PPD, which could then be carefully processed into a new “master batch” that would be known as “PPD-CT68.”[24]
In initially outlining his plan for Ferguson, Landi estimated that from 400 grams of freeze-dried PPD, some two million litres of finished tuberculin PPD could be prepared, which would make possible a total of 20 billion doses. Ultimately, by 1969, Landi was able to prepare 505 grams of PPD-CT68, which would serve as a bio-equivalent standard to PPD-S and also as the principle ingredient of all finished tuberculin PPD produced for Mantoux testing worldwide. This was enough PPD to complete 4.5 billion TB skin tests over the next several decades, most of which supplied directly or indirectly by Connaught.[25] In fact, Landi’s original PPD-CT68 master batch remains in use today, although efforts are currently underway to prepare a new master batch.
Peter Moloney & Unlocking the Mystery of Insulin Rejection

[Sanofi Pasteur Canada Archives]
When Fenje and Landi arrived at Connaught in 1958, they joined one of the Labs’ longest serving researchers, Dr. Peter J. Moloney, who was working on another crucial immunological problem, the mystery surrounding the immunochemistry of insulin and diabetes. While Connaught had been a leading producer of insulin since its discovery in 1921, a key outstanding question was whether insulin was an antigen. As discussed in Articles #3 and #4 of this series, Moloney joined Connaught in 1919 and was instrumental in the development of insulin and diphtheria toxoid. He thus brought a unique immunochemical perspective to the question of whether or not insulin could act as an antigen to stimulate the production of “anti-insulin,” particularly in diabetics dependent upon insulin.

[Canadian Journal od Biochemistry and Physiology 35 (1) (Jan. 1957): 79-92]
As he first described in his 1955 paper, “Antigenicity of Insulin: Diabetes Induced By Specific Antibodies,” working with various experimental animals, Moloney was convinced that “insulin can exercise a true antigenic effect as manifested by anaphylaxis and by the production of neutralizing antibodies.”[26] However, many had been skeptical about this idea since the early days of insulin in the 1920s, suggesting that other factors were at play to explain, in particular, the rare development of immunity to insulin among diabetics.

[Toronto Star, April 2, 1953, p. 5]
In 1957, Moloney made a stronger case in the paper “On the Antigenicity of Insulin,” in which he detailed how insulin was actually a “weak antigen” – at least in comparison with other antigens such as diphtheria or tetanus toxoids. Yet, since insulin was a foreign protein prepared from beef or pork pancreas and administered to diabetics daily over long periods of time, its role as an antigen could have a significant impact.[27] And as he emphasized in his “Antibodies to Insulin” presentation to an international symposium in London in September 1958, Moloney believed that certain diabetics could develop antibodies “which neutralize the physiological action of insulin”; and although this is fortunately encountered rarely in diabetics, these antibodies are “responsible in these [cases] for the ineffectiveness of insulin to control the disease.”[28] As Connaught’s Director, Dr. J.K.W. Ferguson underscored in Connaught’s Annual Report for 1961-62, many diabetics “must have died of this condition in years past when their disease became uncontrollable.”[29] Fortunately, not unlike Fenje and Landi, Moloney was well situated to develop a way at Connaught to resolve this problem, which turned out to be less rare than originally thought.
Moloney’s research had shown that insulin prepared from guinea pig or cod-fish pancreas was much less antigenic and therefore could be of potential value in treating diabetics resistant to regular insulin. However, the effectiveness of such alternate insulin sources was temporary. Taking another approach, Moloney modified regular beef or pork insulin through trying various chemical treatments that might reduce its neutralizability. He soon discovered that treating insulin with sulphuric acid looked promising. The insulin molecule reacted with the sulphuric acid and altered its chemistry in several specific ways that left the treated insulin much less neutralizable by anti-insulin antibodies, which were present in insulin-resistant diabetics.[30]
By 1961, Moloney’s progress with preparing sulphated insulin led to a clinical trial involving an initial group of seven insulin-resistant diabetics. As was reported in the influential 1964 paper, “Sulfated Insulin For Treatment of Insulin-Resistant Diabetics” by Moloney, M.A. Aprile and S. Wilson, the trial’s results proved very encouraging.[31] The clinical trial group had grown to 11 patients, five of whom had successfully relied on sulphated insulin for two years. There was no evidence of the modified insulin acting as an antigen, since the dosage did not increase. Also, in two cases where sulphated insulin treatment was interrupted, the patients were able to re-establish control of their diabetes when sulphated insulin therapy resumed.
Connaught’s “Sulphated Insulin” was approved for use in Canada in 1964 and then in the U.S. as an investigational drug in 1968.[32] Although a highly specialized form of insulin, sulphated insulin played an essential role for insulin-resistant diabetics until 1996. At this point, it was found that newer generation human and synthetic insulins were much less antigenic and that all diabetics could safely take regular insulin. The production of sulphated insulin thus ended.[33]
Moloney’s work on sulphated insulin, along with Fenje’s on rabies vaccine and Landi’s on tuberculin PPD, exemplify the unique type of fluid academic and pragmatic research, innovation, product improvement and public service biologics production work that Connaught excelled in as a vital part of the University of Toronto. It was work that had national, international, and also very personal impacts. However, as the 1960s ended and the 1970s began, Connaught’s unique model based on self-sufficiency within the University of Toronto was becoming increasingly challenging to sustain in a global context of rising regulatory standards and competition. The next article in this series will focus on Connaught’s physical growth during this period, despite these challenges, along with mounting pressures within U of T, and a timely offer, that ultimately led to the sale the Labs in 1972, the proceeds of which creating the Connaught Fund.
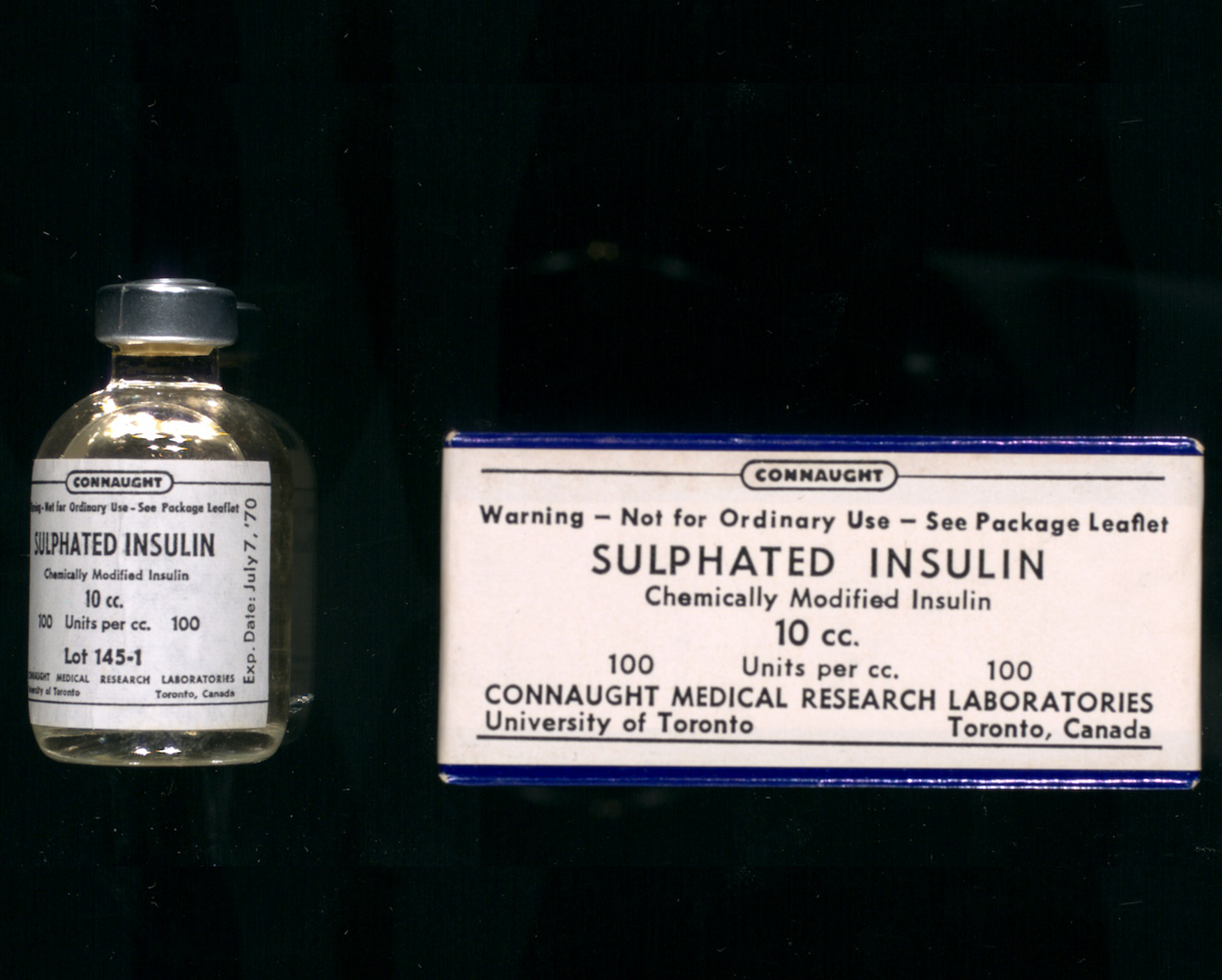
[Sanofi Pasteur Canada Archives]
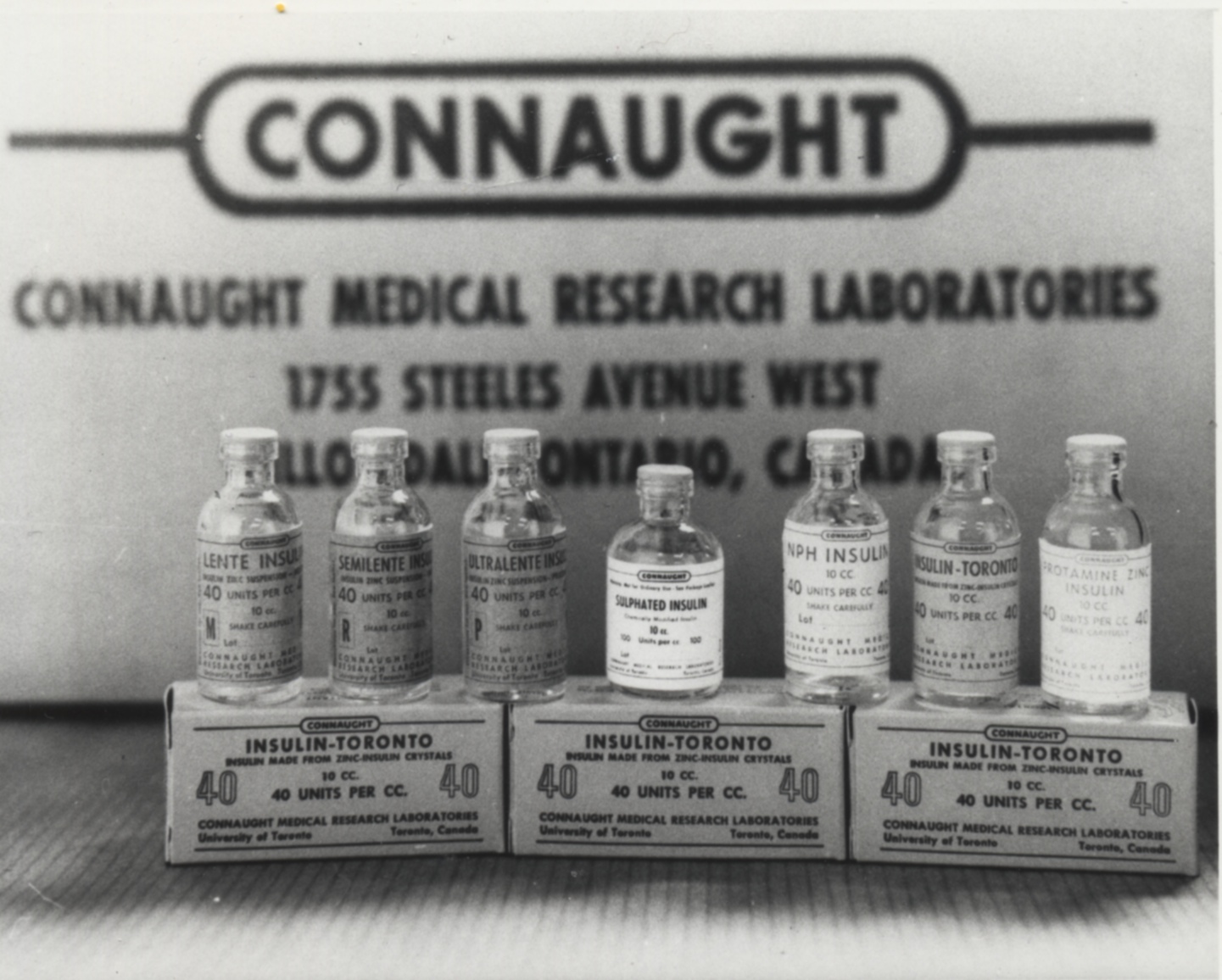
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Useful Resources:
Bator, Paul: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories Limited, Volume 2, 1955-1975; With An Update to the 1990s (Ottawa: Canadian Public Health Association, 1995) Defries, Robert D.: The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (Toronto: University of Toronto Press, 1968) Doctor Peter J. Moloney: Science, Faith Compassion: http://drpetermoloney.com/ Fenje, Paul: “Advances in Rabies Research,” Canadian Journal of Public Health 59 (6) (June 1968): 217-28 Landi, Silvio: “Production and Standardization of Tuberculin” in G.P. Kubica and L.G. Wayne (Eds.), The Mycobacteria: A Sourcebook (New York: Marcel Dekker, 1984): 505-35. Moloney, Mary V.: Behind Insulin: The Life and Legacy of Peter Moloney; A Man’s Catholic Faith and Bold Science (Toronto: Lulu, 2016); book available via http://drpetermoloney.com/ Plotkin, Stanley; Orenstein, Walter; Offit, Paul; Edwards, Kathryn M: Plotkin’s Vaccines (7th edition), (Elsevier, 2018) Rutty, Christopher J. and Sullivan, Susan: This is Public Health: A Canadian History (Canadian Public Health Association, 2010), online eBook: https://www.cpha.ca/history-e-book Sanofi Pasteur Canada, “The Legacy Project”: http://thelegacyproject.ca “Vaccines & Immunization: Epidemics, Prevention & Canadian Innovation: The Online Exhibit, Museum of Health Care, Kingston (2013-14); http://www.museumofhealthcare.ca/explore/exhibits/vaccinations/
Endnotes:
[1] Paul Fenje and C.R. Amies, “The Problem of Rabies in Canada,” Canadian Medical Association Journal 82 (5) (Jan. 30, 1960): 243-45; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1937728/ [2] Christopher J. Rutty, “Canadian Vaccine Research, Production and International Regulation: Connaught Laboratories and Smallpox Vaccines, 1962-1980,” in K. Kroker, J. Keelan and P.M.H. Mazumdar (eds.) Crafting Immunity: Working Histories of Clinical Immunology (Hampshire, UK: Ashgate, 2008), p. 281-82. [3] Fenje and Amies, “The Problem of Rabies in Canada.” [4] D.M. McLean, V.W. Krause, W.M. Wilson, “Rabies Following Skunk Bite,” Canadian Medical Association Journal 82 (Feb. 6, 1960): 315-17; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1937742/ [5] R.D. Defries, The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (University of Toronto Press, 1968), p. 160-61. [6] G.S. Turner, “Rabies Vaccines,” British Medical Bulletin 25 (2) (1969): 136-41. [7] Fenje and Amies, “The Problem of Rabies in Canada,” p. 244. [8] R.E. Kissling, “Growth of Rabies Virus in Non-Nervous Tissue Culture,” Proceedings of the Society for Experimental Biology and Medicine 98 (2) (June 1, 1958): 223-25 [9] Paul Fenje, “Propagation of Rabies Virus in Cultures of Hamster Kidney Cells,” Canadian Journal of Microbiology 6 (5) (Oct. 1960): 479-84; article available at: https://www.nrcresearchpress.com/doi/10.1139/m60-055#.XYpBjJNKgUF [10] Paul Fenje, “A Rabies Vaccine From Hamster Kidney Tissue Cultures: Preparation and Evaluation in Animals,” Canadian Journal of Microbiology 6 (6) (Dec. 1960): 605-09; article available at: http://www.nrcresearchpress.com/doi/10.1139/m60-072 [11] Defries, The First Forty Years, p. 242. [12] Paul A. Bator, Within Reach of Everyone: A History of the University of Toronto School of Hygiene and Connaught Laboratories Limited, Volume II, 1955-1975, with an Update to the 1990s (Ottawa: Canadian Public Health Association, 1995), p. 96, 122. [13] L. Pinteric, P. Fenje, J.D. Almeida, “The Visualization of Rabies Virus in Mouse Brain,” Virology 20 (1) (May 1963): 208-11; Connaught Medical Research Laboratories, Annual Report, 1961-62, p. 8-9, Sanofi Pasteur Canada Archives. [14] Paul Fenje and L. Pinteric, “Potentiation of Tissue Culture Rabies Vaccine by Adjuvants,” American Journal of Public Health 56 (12) (Dec. 1966): 2106-13; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1257371/ [15] Connaught Medical Research Laboratories, Annual Report, 1969-70, p. 9, Sanofi Pasteur Canada Archives. [16] M.K. Abelseth, “An Attenuated Rabies Vaccine for Domestic Animals Produced in Tissue Culture,” The Canadian Veterinary Journal 5 (11) (Nov. 1964): 279-86; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1695899/ [17] Connaught Medical Research Laboratories, Annual Report, 1965-66, p. 12, Sanofi Pasteur Canada Archives; M.K. Abelseth, “Further Studies on the use of ERA Rabies Vaccine in Domestic Animals,” The Canadian Veterinary Journal 8 (10) (Oct. 1967): 221-27; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1696964/ [18] Elsie Lee and Robert S. Holzman, “Evolution and Current Use of the Tuberculin Test,” Clinical Infectious Diseases 34 (3) (Feb. 1, 2002): 365-70; article available at: https://academic.oup.com/cid/article/34/3/365/389398; S. Landi, “Tuberculin,” Contox (Connaught Laboratories Employee Newsletter), No. 14 (Feb. 1969), p. 1-2, Sanofi Pasteur Canada Archives; article available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-11/CONTOX-1969-02-n14-TB-tuberculin-insulin.pdf) [19] Defries, The First Forty Years, p. 118. [20] H. Yang, N.A Kruch-Garcia, K.M. Dobos, “Purified Protein Derivatives of Tuberculin: Past, Present, and Future,” FEMS Immunol Med Microbiol 66 (3) (Dec. 2012): 273-80; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3491170/) [21] Landi, “Tuberculin,” Contox, No. 14 (Feb. 1969), p. 1-2. [22] S. Landi, “Preparation, Purification, and Stability of Tuberculin,” Applied Microbiology 11 (5) (Sept. 1963): 408-12; article available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1058016/) [23] J.C.W. Weber and W.R. Ashford, “New Drug Development Program: Connaught Medical Research Laboratories,” March 16, 1972, pp. 11-14; Sanofi Pasteur Canada Archives, CLL General, 1971-73, Box 29; document available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-11/Weber-Ashford-CMRL-NewDrugDevelopmentProgram-1972-03-16-SPCA-CLLgeneral1971-73-Box29.pdf [24] Memos between J.K.W. Ferguson and Silvio Landi, April 11-16, 1968, Sanofi Pasteur Canada Archives, Box AT001501156. [25] Connaught Medical Research Laboratories, Annual Report, 1970-71, p. 7-8, Sanofi Pasteur Canada Archives; available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-11/Tuberculin-PPD-CMRLAnnualReport1970-71-p7-8.pdf; S. Landi and H.R. Held, “Preparation and Characterization of a Large Batch of Tuberculin Purified Protein Derivative (PPD-CT68), Annali Sclavo 22 (6) (1980): 899-907 [26] P.J. Moloney and M. Coval, “Antigenicity of Insulin” Diabetes Induced by Specific Antibodies,” Biochemical Journal 59 (2) (Feb. 1955): 179-85. [27] P.J. Moloney and L. Goldsmith, “On the Antigenicity of Insulin,” Canadian Journal of Biochemistry and Physiology 35 (1) (Jan. 1957): 79-92; article available at: http://www.nrcresearchpress.com/doi/abs/10.1139/o57-011) [28] P.J. Moloney, “Antibodies to Insulin,” in W.A. Brown and F.W. Wolff (eds), The Mechanism of Action of Insulin: A Symposium, London, September 1958 (published in 1960), p. 201-09; reprint held in Sanofi Pasteur Canada Archives; scan available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-11/Moloney-PJ-AntibodiesToInsulin-Symposium-London-1958-09-pub1960-p201.pdf) [29] Connaught Medical Research Laboratories, Annual Report, 1961-62, p. 7-8, Sanofi Pasteur Canada Archives. [30] Peter Moloney, Research Notes, Sulphated Insulin, 1961-62, Sanofi Pasteur Canada Archives, 83-010-28. [31] P.J. Moloney, M.A. Aprile, S. Wilson, “Sulfated Insulin for Treatment of Insulin-Resistant Diabetics,” The Journal of New Drugs 4 (5) (Sept-Oct. 1964): 258-63. See also P.J. Moloney, “Insulin as an Antigen,” Modern Medicine of Canada 26 (Dec. 1971): 23-25; reprint held in Sanofi Pasteur Canada Archives; scan available at: http://healthheritageresearch.com/clients/docs/UTCF/Article-11/Moloney-PJ-InsulinAsAnAntigen-ModernMedicineOfCanada-1971-12-v26-p23.pdf) [32] J.K. Davidson and D.W. DeBra, “Immunological Insulin Resistance,” Diabetes 27 (3) (March 1978): 307-18. [33] A. Carpentier, J. Whither, B. Vukusic, K. Lawday, A.H. Boss, G.F. Lewis, “An Epitaph for Sulfated Insulin,” Diabetes Care 21 (9) (Sept. 1998): 1571-72.
