After an especially productive decade during the 1930s, particularly with insulin and heparin development, as was recounted in Article #5 in this series, the start of World War II began an intense period of biological health products research, innovation and production at Connaught Laboratories focused on supporting the war effort. When Canada entered the war on September 10, 1939, the Labs had a staff of 252, and when it ended on August 14, 1945, there were 800, although that number peaked at about 1,500 in 1944. There were 96 staff members who served in the military during the war and of this group, two made the ultimate sacrifice.

[Globe & Mail, June 22, 1940, page 8]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
[Photo by author]
Connaught’s founder and Director, Dr. John G. FitzGerald, also made the ultimate sacrifice. But his sacrifice was not on the fields of combat, but caused by personal mental health struggles. The late 1930s had been an especially intense time for FitzGerald. In addition to leading Connaught and the School of Hygiene, he served as U of T’s Dean of Medicine and as Scientific Director of the Rockefeller Foundation, travelling through Europe to assess dozens of medical schools and hospitals. His additional responsibilities exacerbated his growing mental health struggles, leading to a first suicide attempt in February 1939. After spending time in a private sanatorium he tried to resume his work, but on June 16, 1940, a second failed attempt to end his life put FitzGerald into Toronto General Hospital. On June 20, his determination ultimately proved tragically successful. FitzGerald’s suicide was never publicly acknowledged (the official cause of death was a duodenal ulcer) until his grandson, James FitzGerald, was driven to solve his family’s “self-murder mystery” in the award-winning book, What Disturbs Our Blood, published in 2010.

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Dr. FitzGerald’s tragic passing left Dr. Robert D. Defries as Director of Connaught and the School of Hygiene, although he had effectively been in charge through much of the 1930s. Defries’ practical managerial skills, as much as his scientific, biomedical and public health expertise, were a major asset when the war started and the Labs were asked by the Canadian military to supply existing products, such as smallpox vaccine and diphtheria toxoid on a large scale, and to develop and supply several new biological health products while also meeting the Labs’ civilian demands for public health products. During World War I, tetanus had been one of the most serious health threats to soldiers and the preparation of tetanus antitoxin was central to Connaught’s contribution to the war effort. But tetanus antitoxin only treated the disease; it provided very limited immunity. Soon after the discovery of diphtheria toxoid in the early 1920s, as described in Article #4 in this series, Gaston Ramon at the Pasteur Institute in France applied a similar method to prepare tetanus toxoid, which worked like a vaccine to prevent the disease. Connaught began development work on tetanus toxoid in 1927, but it was not until the start of World War II that the Labs prepared the toxoid on a large scale. However, it soon became clear that while the toxoid was effective, reports of occasional undesirable reactions prompted an intensive research effort at Connaught to eliminate such reactions. Led by Dr. Edith M. Taylor, this work focused on developing an improved method for the culturing of tetanus toxin, from which the toxoid was prepared. Taylor’s method, which utilized a more carefully prepared culture media that she devised, rather than the standard commercially available media, produced a toxin that surpassed the potency of other methods and resulted in a toxoid free of any undesirable effects. Dr. Taylor’s work with tetanus toxoid, which followed her similar bacterial culture media work with diphtheria toxoid and pertussis vaccine, led to her being awarded the Order of the British Empire at the end of the war. A major infectious disease threat strongly associated with war was typhus, which is a group of bacterial diseases that include epidemic typhus, scrub typhus and murine typhus. Epidemic typhus has historically been most common and caused by the Rickettsia prowazeki bacterium and spread via body lice. Responsible for much misery and death, especially during the 19th century, epidemic typhus would ravage the armies of the Eastern Front during World War I, although delousing stations for troops on the Western Front helped minimize incidence. Typhus fatalities were generally between 10 and 40% of those infected, with those nursing the sick especially vulnerable.

[Sanofi Pasteur Canada Archives]

[Canadian Journal of Public Health, 34 (Sept. 1943): 406-14]

[Globe & Mail, July 6, 1945, page 11.]
When World War II started there was no typhus vaccine available, but encouraging research, particularly at the United States Public Health Service, and then at the Harvard School of Public Health, opened the door to a vaccine based on cultivating the rickettsia bacterium in fertile, developing hen’s eggs, or in chick-embryo tissue. Led by Dr. James Craigie, Connaught launched an intensive typhus research program in July 1940 with funding from the National Research Council of Canada. Craigie initially focused on studying the USPHS and Harvard methods and realized that two key problems remained: much richer bacterial cultures were needed, as was a better purification method. A more detailed understanding of the growth properties of the rickettsiae organism helped solve the first problem, while the second problem was resolved by the development of a precise ether-based method by Craigie, which could differentiate the growing rickettsiae organism from other materials in egg-yolk, resulting in purer cultures for vaccine production. By August 1942, a large-scale typhus vaccine production program was initiated, the cultivation process overseen by Dr. Laurella McClelland, which involved inoculating some 2,000 fertile eggs each day. The ether-based purification process was supervised by Dr. Raymond Parker, and from January 1943 vaccine production rose steadily until the end of the war, with 1,000,000 doses produced each month during peak production. The vaccine was also made available to Great Britain and the United States to immunize troops in all theatres of war. After the war, Dr. Craigie’s pioneering typhus vaccine work was recognized with his receipt of the United States of America Typhus Commission Medal.
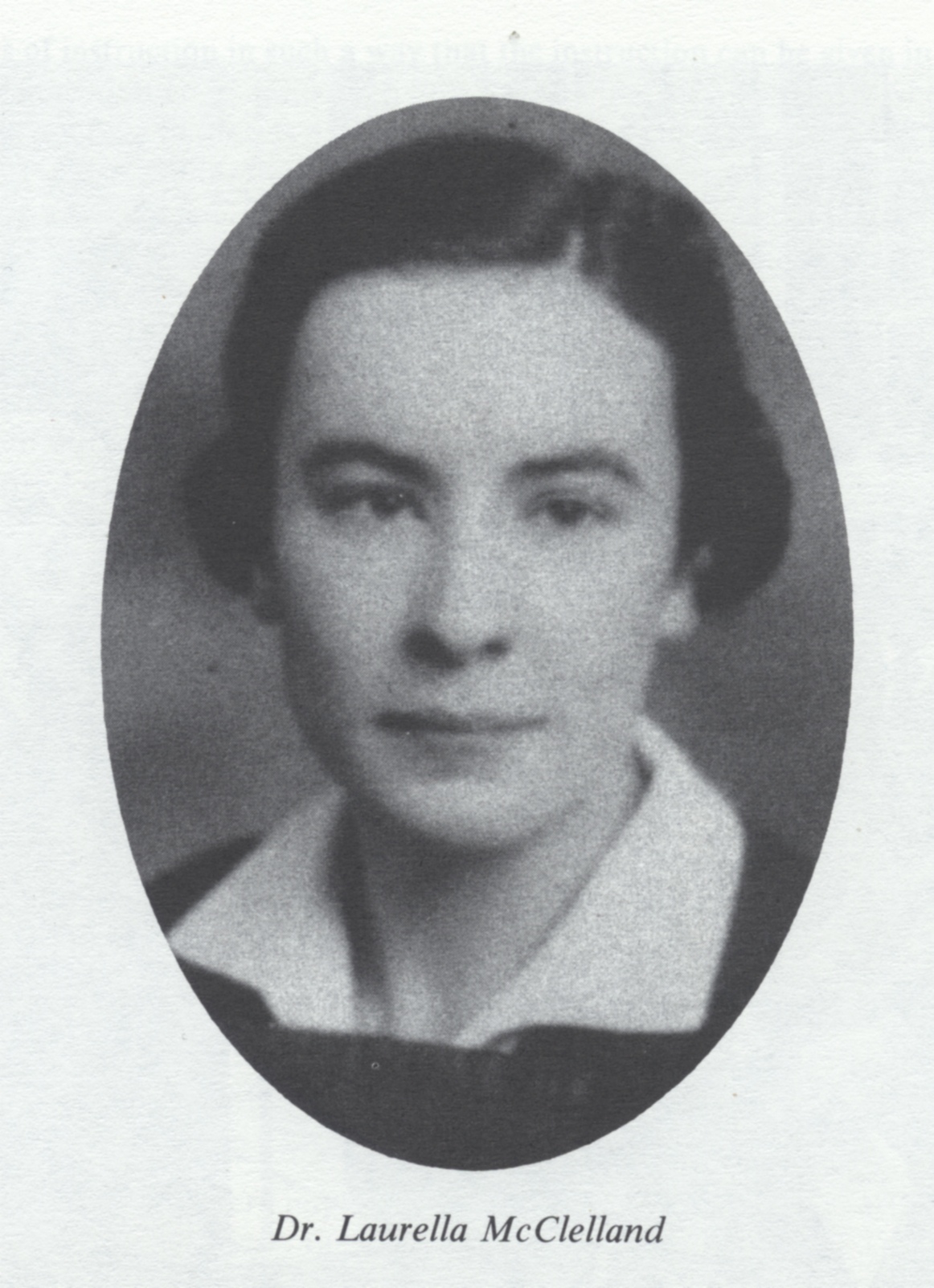
[P.A. Bator, Paul with Andrew J. Rhodes: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories, Volume 1, 1927-1955 (Ottawa: CPHA, 1990), page 83]
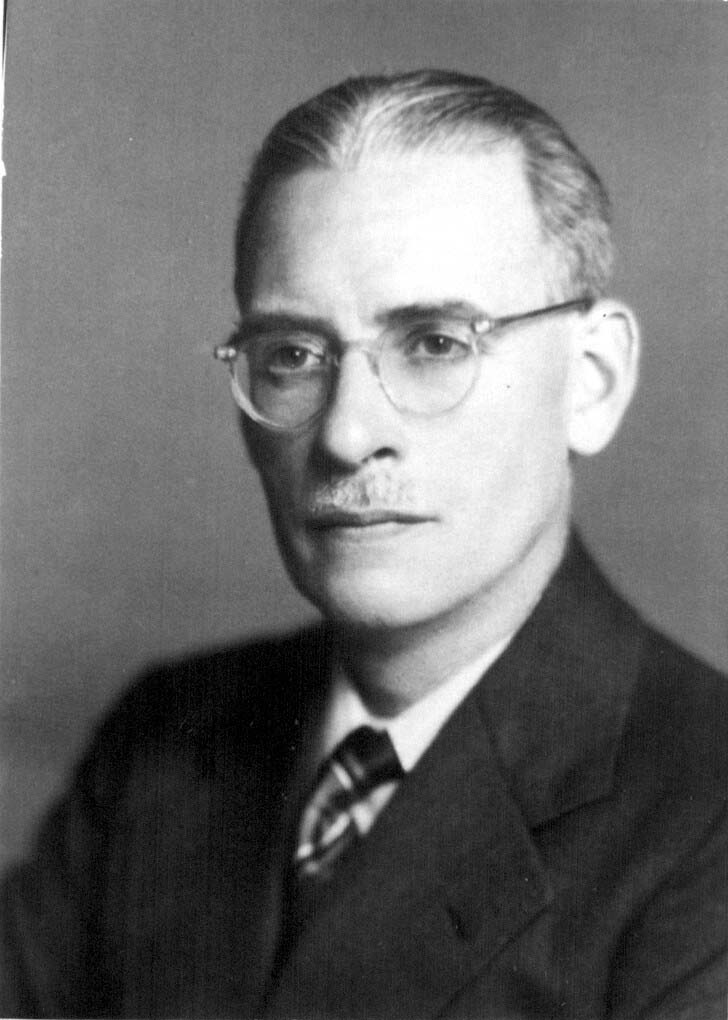
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
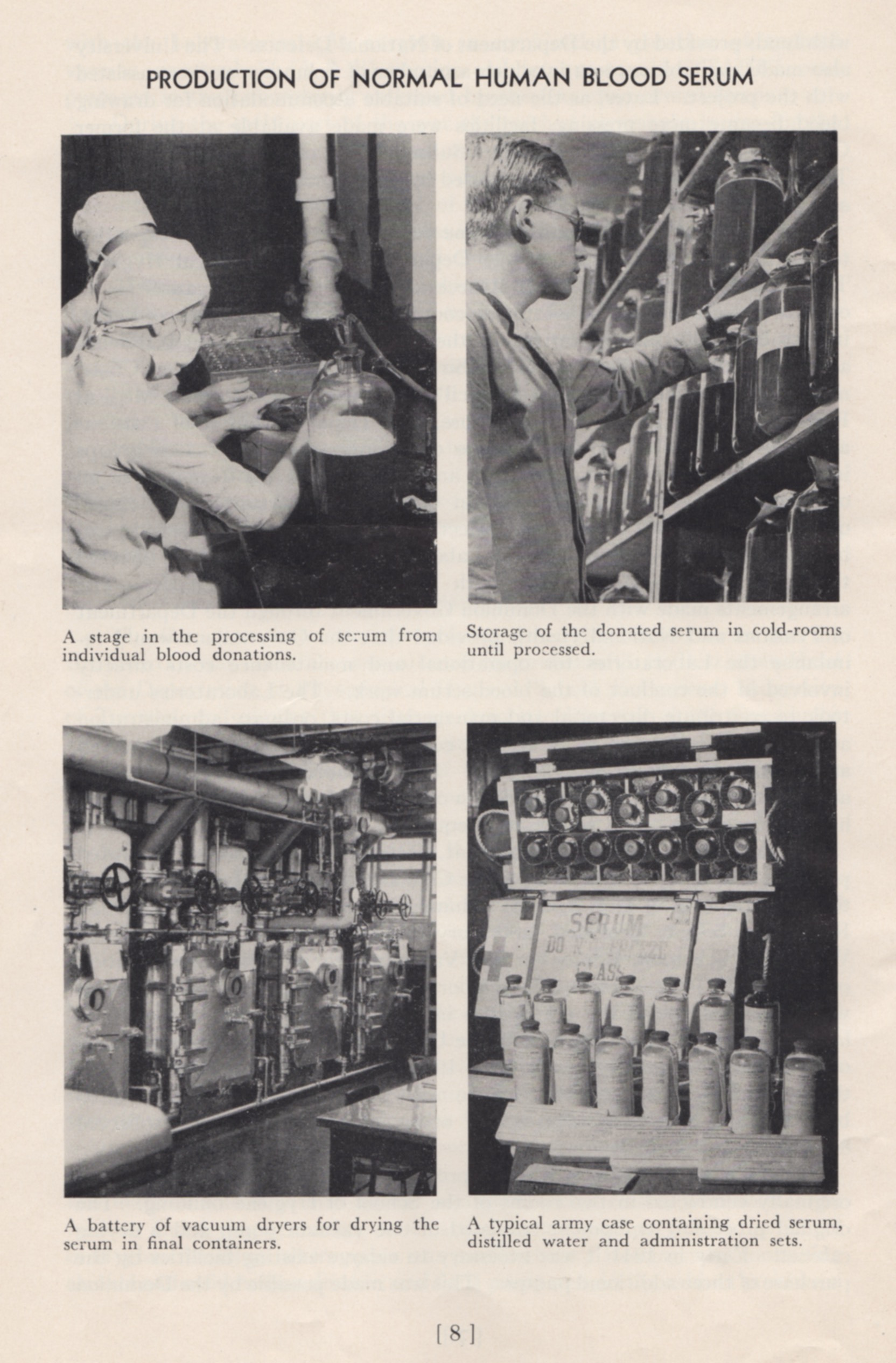
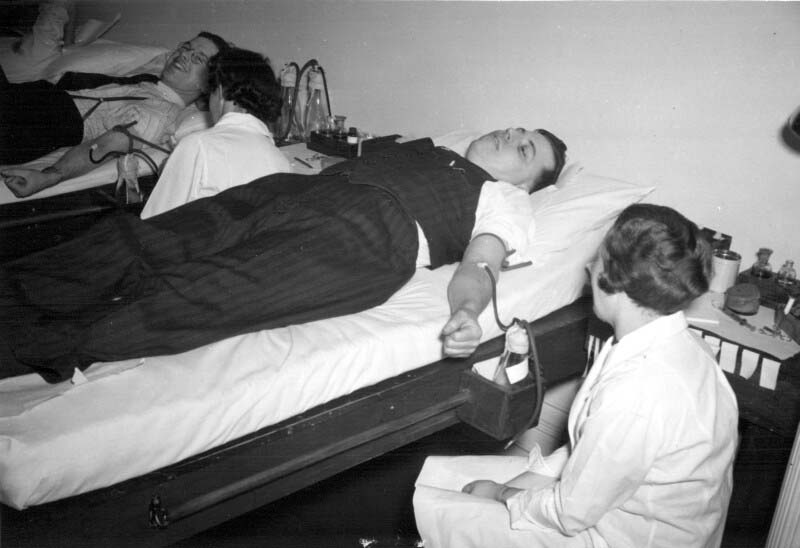
When World War II started, it was clear to Dr. Charles Best that the Canadian military would need a larger, safer and better-preserved supply of blood at the front than was the case during World War I. While blood transfusions saved the lives of many soldiers during the Great War, by the 1930s there were better alternatives to using whole blood in the treatment of wounded soldiers. Best was then Head of the Department of Physiological Hygiene at the School of Hygiene and also an Associate Director at Connaught, and he led an initiative to prepare dried blood serum in collaboration with the National Research Council, the Department of National Defense and the Canadian Red Cross. Beginning modestly in September 1939 with the recruitment of blood donors among School and Connaught staff, and then U of T students, and using a small workbench in the School to process the blood, the effort grew quickly. After the blood was drawn, it was typed and allowed to clot and then the serum was separated from the clotted blood and the various sera combined into groups based on blood type. After passing sterility tests, the freeze-drying process was conducted in a small room in the School. By October 1940, a larger coordinated effort for blood processing was needed. In January 1941 Connaught took a leadership role in processing blood collected through a national Red Cross donor program and then preparing the freeze-dried serum. Larger facilities were soon required, forcing some of the Labs’ peacetime operations to stop or be crowded together to make sufficient room. With more than 11,000 blood donations received monthly by March 1942, even more processing space was needed. By October, this increased to over 57,000 donations received monthly and further expansion was urgently needed. In August 1943, the large vacant building in Spadina Circle, originally Knox College, was acquired by the Labs and quickly renovated to accommodate expanded dried blood serum production. By the end of the war, Connaught had received over 2,250,000 blood donations, which made it possible to prepare some 500,000 bottles of dried serum. Most of the dried blood serum was sent to England where it was packed with bottles of distilled water necessary for dissolving the dried serum prior to its use to treat wounded soldiers.
[Sanofi Pasteur Canada Archives]
[Sanofi Pasteur Canada Archives]

Dr. Charles H. Best initiated the Canadian Blood Serum Project when the war started and in 1942 was appointed director of the medical research unit of the Royal Canadian Navy, with the rank of Surgeon, Lieutenant-Commander; in 1943, he was promoted to Surgeon Captain.
[Sanofi Pasteur Canada Archives]

[Globe & Mail, Dec. 6, 1943, page 5]

[Sanofi Pasteur Canada Archives]
While also driven by the urgent need for more space to prepare dried blood serum, Connaught’s acquisition of the Spadina Building in August 1943 was also to accommodate the Labs’ largest and most time-sensitive wartime project, the large-scale production of “wonder drug” penicillin, in time for the long-planned “D-Day” invasion of occupied France in June 1944. Penicillin was discovered by accident in 1928 by Alexander Fleming when he noticed that bacterial growth on culture plates was being inhibited by growths of airborne moulds that had fallen on the plates. The mould, which he identified as a “penicillium,” seemed to produce an antibiotic material. However, progress in cultivating penicillin was slow and its potential medical use in treating certain infections was not clear until 1940. Fuelled by the war, penicillin development accelerated in 1941 with several researchers at U of T based in the Banting Institute and the Department of Bacteriology, who were making contributions to the refinement of penicillin extraction methods. In 1943, Canadian military demand for penicillin fuelled a heroic effort at Connaught to prepare the drug on a large scale, despite not having a suitable building, or a clear idea about how to proceed. However, after much searching, a large, vacant and convenient site was finally found in the Spadina Building, the Department of Defense helping pay for its acquisition and extensive renovation. Wartime shortages of materials and human resources certainly added to the challenges faced by Dr. Defries, who oversaw what was effectively a major military operation at the Labs. The original production method involved growing penicillin-infected mould in large milk bottles in cool temperature-controlled incubator rooms. Some 250,000 bottles were required and the only place they could be stored was under the stands of the university’s football field. During the height of production, 30,000 bottles were utilized every 24 hours, 7 days a week. Some 1,000 gallons of “mould juice” was produced daily and extracting the penicillin was not unlike separating cream from milk. On April 26, 1944, the first penicillin came off the production line and by May 20, the Canadian government approved the first vials of the dirty yellow penicillin powder. Some 17 days later, with penicillin packed in their medical equipment, the allied D-Day troops landed in France.

[Sanofi Pasteur Canada Archives]
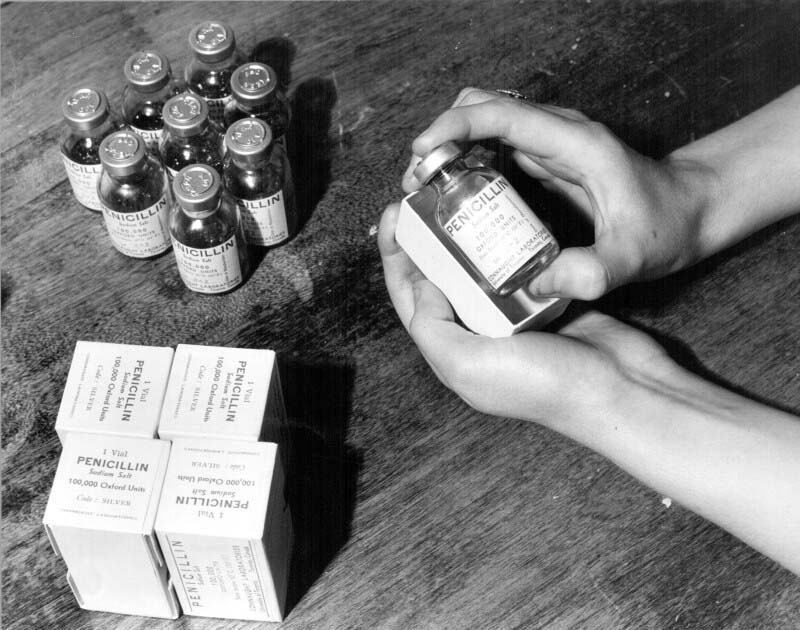
[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]

[Sanofi Pasteur Canada Archives]
Several types of the clostridia bacterium, such as tetanus during World War I, are widely distributed in nature through the intestinal tract of animals, causing disease in humans when exposed to dormant spores of the bacterium. Such exposure and frequently deadly infections, known as gas-gangrene, were exacerbated in the wartime conditions of World War II. Early in the war, Connaught was able to prepare a gas-gangrene antitoxin, although there had initially been limited demand. However, in May 1943 the Canadian military asked Connaught for 150,000 doses, a demand that surged when a mixture of gas-gangrene antitoxins proved effective in combination with surgery and new drugs. The challenge for Connaught was accommodating the 300+ horses that would be needed to prepare the antitoxin on this scale, when the Labs’ farm site had facilities for only 100. Similar to preparing diphtheria and tetanus antitoxins, horses were given a series of increasingly large doses of the gas-gangrene toxin over several weeks. The horses were not harmed and after blood tests showed they had developed immunity to the toxin, some blood was taken from each and the antitoxin prepared from the white blood cells. Thus, to meet the high demand for the antitoxin, during the summer of 1943 six new stables were built, along with a new operating building, an expanded concentration building and a greatly enlarged water supply. Dr. Edith Taylor would play a key role in the success of the project by developing new methods for large-scale purification and concentration of the antitoxin. In the end, Connaught would supply some 300,000 doses of gas-gangrene antitoxin, which would involve 1,000 horses, with many also accommodated at the Hamilton Jockey Club.

[Canadian Journal of Public Health, 34 (Oct. 1943), p. 474]
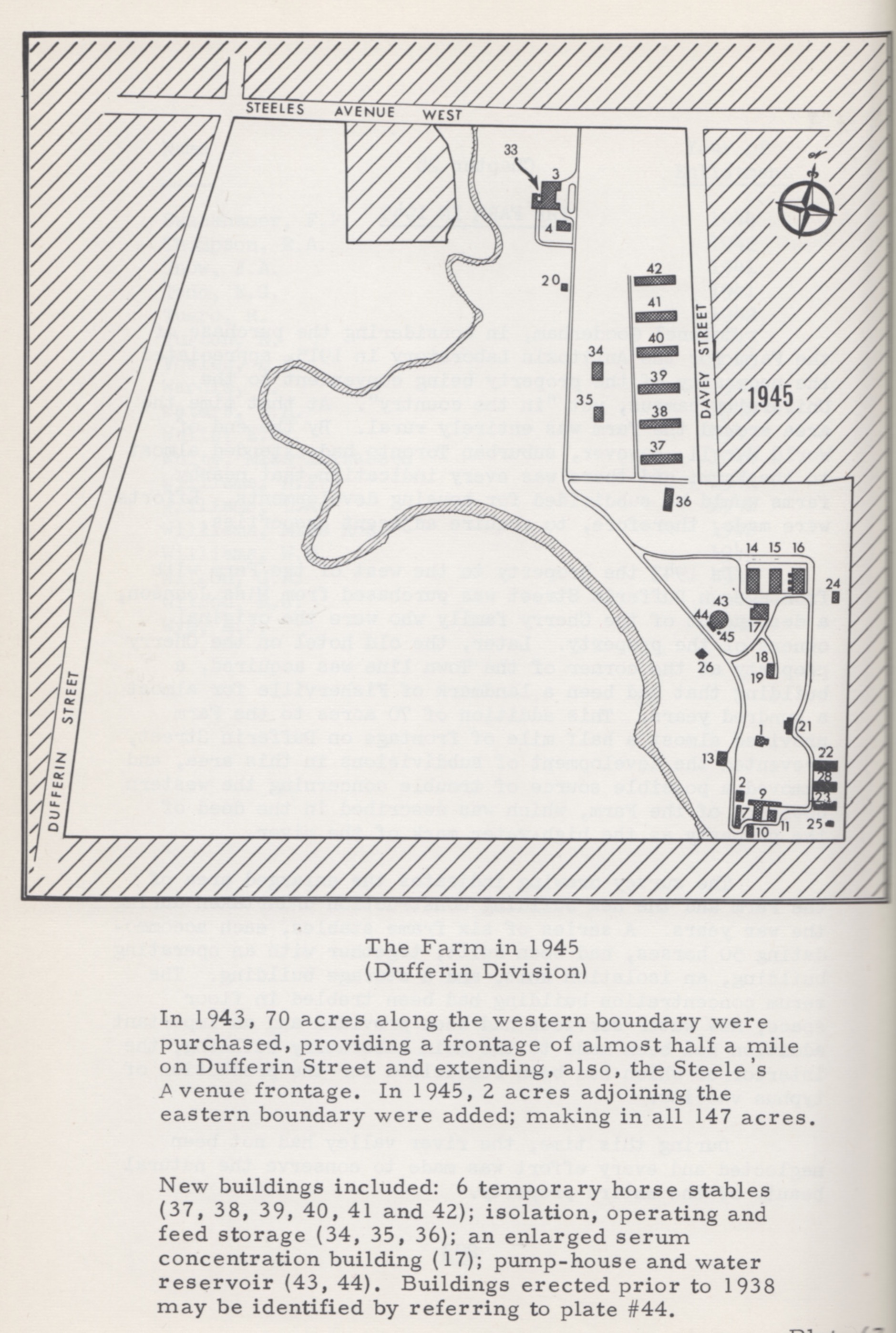
[R.D. Defries, The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (U of T Press, 1968), p. 204]
As described in Article #2 of this series, the “Spanish Flu” pandemic at the end of World War I had prompted Connaught to prepare an influenza vaccine based on the prevailing, but erroneous, assertion that the disease was caused by a type of influenza bacterium. However, by the time World War II started it was clear that influenza was caused by two distinct strains of the influenza virus, A and B, which could be cultivated in various chick-embryo tissues to prepare a vaccine, particularly the allantonic fluid that surrounds the embryo. Led by Dr. Ronald Hare prior to the war, Connaught had initiated influenza virus studies and established a centre in Canada for the isolation of influenza virus strains. Building on her experience with typhus vaccine production, Dr. Laurella McClelland would lead Connaught’s influenza vaccine development, production and testing, the work accelerating in 1944 after the Canadian military asked Connaught to supply vaccine for the soldiers. Between August 1944 and March 1945, some 36 staff members processed about 2,000 eggs daily to produce a total of 200,000 doses of influenza vaccine. By the end of World War II, the rapidly growing public health threat of another virus, the poliovirus, and intense efforts to prepare a vaccine to prevent “the crippler” would quickly dominate the post-war research work of Connaught Labs and lead to fundamental innovations that made possible an effective polio vaccine. That story will be the focus of the next article in this series.
Useful Resources:
Bator, Paul with Andrew J. Rhodes: Within Reach of Everyone: A History of the University of Toronto School of Hygiene and the Connaught Laboratories, Volume 1, 1927-1955 (Ottawa: Canadian Public Health Association, 1990) Defries, Robert D: Wartime Work, 1939-1945, Connaught Medical Research Laboratories, University of Toronto (Booklet, 1945); complete booklet available at: http://healthheritageresearch.com/clients/docs/SPCA/CMRL-WartimeWork-1939-1945-booklet.pdf Defries, Robert D.: The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto (University of Toronto Press, 1968) FitzGerald, James: What Disturbs Our Blood: A Son’s Quest to Redeem the Past (Toronto: Random House, 2010); details at: http://www.jamesfitzgerald.info Rutty, Christopher J. and Sullivan, Susan: This is Public Health: A Canadian History (Canadian Public Health Association, 2010), online eBook: https://www.cpha.ca/history-e-book Sanofi Pasteur Canada, “The Legacy Project”: http://thelegacyproject.ca “Vaccines & Immunization: Epidemics, Prevention & Canadian Innovation: The Online Exhibit, Museum of Health Care, Kingston (2013-14); http://www.museumofhealthcare.ca/explore/exhibits/vaccinations/ “Within Reach of Everyone: The Birth, Maturity & Renewal of Public Health at the University of Toronto,” Dalla Lana School of Public Health website feature: http://www.dlsph.utoronto.ca/history/
